Page 122 - Gas Adsorption Equilibria
P. 122
108 Chapter 2
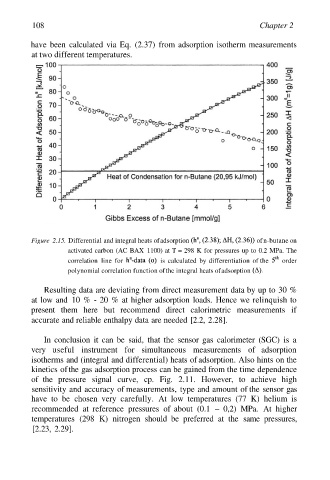
have been calculated via Eq. (2.37) from adsorption isotherm measurements
at two different temperatures.
Figure 2.15. Differential and integral heats of adsorption of n-butane on
activated carbon (AC BAX 1100) at T = 298 K for pressures up to 0.2 MPa. The
correlation line for is calculated by differentiation of the order
polynomial correlation function of the integral heats of adsorption
Resulting data are deviating from direct measurement data by up to 30 %
at low and 10 % - 20 % at higher adsorption loads. Hence we relinquish to
present them here but recommend direct calorimetric measurements if
accurate and reliable enthalpy data are needed [2.2, 2.28].
In conclusion it can be said, that the sensor gas calorimeter (SGC) is a
very useful instrument for simultaneous measurements of adsorption
isotherms and (integral and differential) heats of adsorption. Also hints on the
kinetics of the gas adsorption process can be gained from the time dependence
of the pressure signal curve, cp. Fig. 2.11. However, to achieve high
sensitivity and accuracy of measurements, type and amount of the sensor gas
have to be chosen very carefully. At low temperatures (77 K) helium is
recommended at reference pressures of about (0.1 – 0,2) MPa. At higher
temperatures (298 K) nitrogen should be preferred at the same pressures,
[2.23, 2.29].