Page 153 - Gas Adsorption Equilibria
P. 153
3. Gravimetry 139
sorbent was cooled from 323 K to 298 K. This effect was irreversible, i. e. this
amount of helium was sorbed permanently in the sorbent and for other
experiments could be considered as (constant) “presorbed gas” [3.30]. This
effect of a spontaneous and irreversible uptake of helium at ambient or near
ambient temperatures was observed in all kinds of sorbent materials, i. e.
activated carbons, zeolites, metal foams and others, the specific amount of
helium adsorbed depending strongly on the pretreatment of the sorbent
material, i. e. its activation procedure etc., cp. Chap. 1, Sect. 4.2.
In Figure 3.10 equilibrium adsorption data of pure gases CO,
on activated carbon NORIT Rl EXTRA at pressures and
temperatures 298.15 K are shown [3.27], [3.28]. The mol numbers of Gibbs
excess amounts adsorbed per unit mass of sorbent are depicted as function of
sorptive’s gas pressure (and temperature). Relative uncertainties of data are
about Measurements were performed with a two beam
balance (Sartorius 4104S), cp. Fig. 3.2. Prior to all measurements the sorbent
was activated by the “standard procedure”, i. e. by heating it up to 423 K for
(4-5) h in vacuum
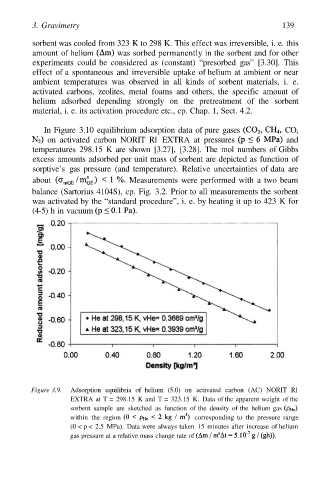
Figure 3.9. Adsorption equilibria of helium (5.0) on activated carbon (AC) NORIT Rl
EXTRA at T = 298.15 K and T = 323.15 K. Data of the apparent weight of the
sorbent sample are sketched as function of the density of the helium gas
within the region corresponding to the pressure range
(0 < p < 2.5 MPa). Data were always taken 15 minutes after increase of helium
gas pressure at a relative mass change rate of