Page 157 - Gas Adsorption Equilibria
P. 157
3. Gravimetry 143
Adsorption Theory (IAST) approximation [3.38]. In this theory the selectivity
of two gases simply can be represented as ratio of the Henry-constants
i. e. the steepnesses of the two pure gas adsorption isotherms at
In our case we have
i. e. for T = 293 K, Köstrolith SX6 is a zeolite molecular sieve of
LiNaX-type with a (low) atomic ratio of Its BET surface
measured with (5.0) at 77 K is The specific volume of macro-
and (partly) mesopores determined by Hg-porosimetry is The
specific volume of the micropores determined by adsorption of at 273 K
and calculated by the Dubinin-Astarkhov-method [3.52] is The
so-called helium volume of dry zeolite SX6 has been determined
gravimetrically after activating the zeolite at 700 K for 6 hours as
[3.16].
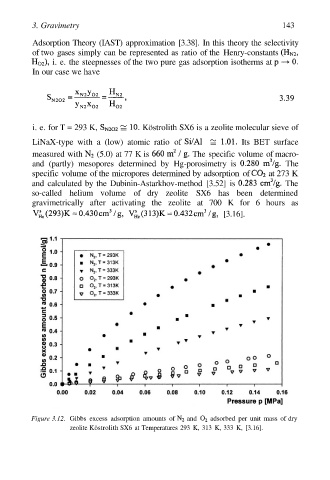
Figure 3.12. Gibbs excess adsorption amounts of and adsorbed per unit mass of dry
zeolite Köstrolith SX6 at Temperatures 293 K, 313 K, 333 K, [3.16].