Page 159 - Gas Adsorption Equilibria
P. 159
3. Gravimetry 145
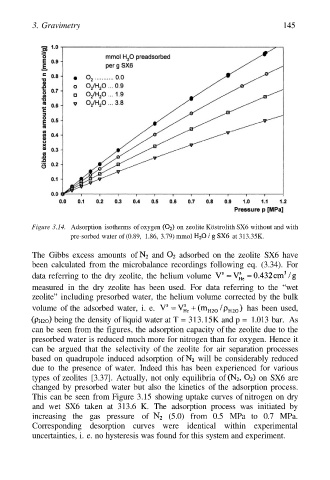
Figure 3.14. Adsorption isotherms of oxygen on zeolite Köstrolith SX6 without and with
pre-sorbed water of (0.89, 1.86, 3.79) mmol at 313.35K.
The Gibbs excess amounts of and adsorbed on the zeolite SX6 have
been calculated from the microbalance recordings following eq. (3.34). For
data referring to the dry zeolite, the helium volume
measured in the dry zeolite has been used. For data referring to the “wet
zeolite” including presorbed water, the helium volume corrected by the bulk
volume of the adsorbed water, i. e. has been used,
being the density of liquid water at T = 313.15K and p = 1.013 bar. As
can be seen from the figures, the adsorption capacity of the zeolite due to the
presorbed water is reduced much more for nitrogen than for oxygen. Hence it
can be argued that the selectivity of the zeolite for air separation processes
based on quadrupole induced adsorption of will be considerably reduced
due to the presence of water. Indeed this has been experienced for various
types of zeolites [3.37]. Actually, not only equilibria of on SX6 are
changed by presorbed water but also the kinetics of the adsorption process.
This can be seen from Figure 3.15 showing uptake curves of nitrogen on dry
and wet SX6 taken at 313.6 K. The adsorption process was initiated by
increasing the gas pressure of (5.0) from 0.5 MPa to 0.7 MPa.
Corresponding desorption curves were identical within experimental
uncertainties, i. e. no hysteresis was found for this system and experiment.