Page 163 - Gas Adsorption Equilibria
P. 163
3. Gravimetry 149
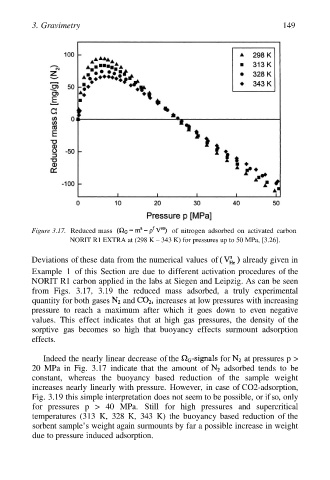
Figure 3.17. Reduced mass of nitrogen adsorbed on activated carbon
NORIT R1 EXTRA at (298 K – 343 K) for pressures up to 50 MPa, [3.26].
Deviations of these data from the numerical values of already given in
Example 1 of this Section are due to different activation procedures of the
NORIT R1 carbon applied in the labs at Siegen and Leipzig. As can be seen
from Figs. 3.17, 3.19 the reduced mass adsorbed, a truly experimental
quantity for both gases and increases at low pressures with increasing
pressure to reach a maximum after which it goes down to even negative
values. This effect indicates that at high gas pressures, the density of the
sorptive gas becomes so high that buoyancy effects surmount adsorption
effects.
Indeed the nearly linear decrease of the for at pressures p >
20 MPa in Fig. 3.17 indicate that the amount of adsorbed tends to be
constant, whereas the buoyancy based reduction of the sample weight
increases nearly linearly with pressure. However, in case of CO2-adsorption,
Fig. 3.19 this simple interpretation does not seem to be possible, or if so, only
for pressures p > 40 MPa. Still for high pressures and supercritical
temperatures (313 K, 328 K, 343 K) the buoyancy based reduction of the
sorbent sample’s weight again surmounts by far a possible increase in weight
due to pressure induced adsorption.