Page 161 - Gas Adsorption Equilibria
P. 161
3. Gravimetry 147
As it was not possible to correlate experimental data of Fig. 3.15 using a
single relaxation time model we argue that the uptake of nitrogen in the
zeolite is a two step process. In the first step the are quickly
entering the macropores and also some mesopores In a second step the
molecules slowly diffuse from the macropores to the micropores
Presorption of water changes the first mechanism, i. e. uptake of in the
wide pores is somewhat hindered by the water molecules. Contrary to this the
secondary adsorption mechanism, i. e. transfer of to the
micropores does not seem to be much influenced by the presence of the
permanently presorbed small amounts of water [3.16]. * ) To give another
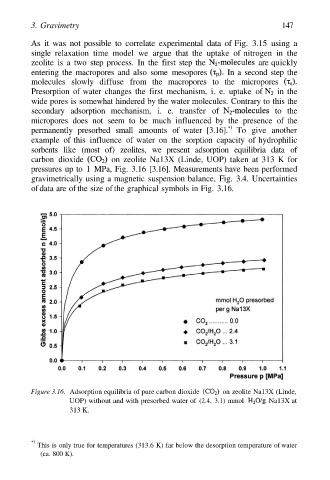
example of this influence of water on the sorption capacity of hydrophilic
sorbents like (most of) zeolites, we present adsorption equilibria data of
carbon dioxide on zeolite Na13X (Linde, UOP) taken at 313 K for
pressures up to 1 MPa, Fig. 3.16 [3.16]. Measurements have been performed
gravimetrically using a magnetic suspension balance, Fig. 3.4. Uncertainties
of data are of the size of the graphical symbols in Fig. 3.16.
Figure 3.16. Adsorption equilibria of pure carbon dioxide on zeolite Na13X (Linde,
UOP) without and with presorbed water of (2.4, 3.1) mmol Na13X at
313 K.
* )
This is only true for temperatures (313.6 K) far below the desorption temperature of water
(ca. 800 K).