Page 165 - Gas Adsorption Equilibria
P. 165
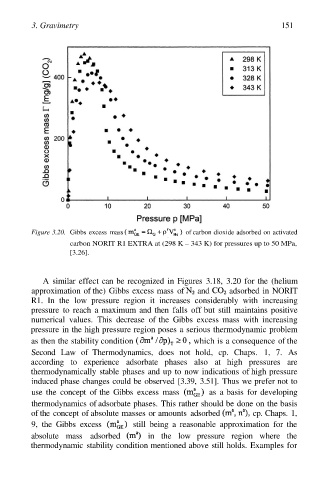
3. Gravimetry 151
Figure 3.20. Gibbs excess mass of carbon dioxide adsorbed on activated
carbon NORIT R1 EXTRA at (298 K – 343 K) for pressures up to 50 MPa,
[3.26].
A similar effect can be recognized in Figures 3.18, 3.20 for the (helium
approximation of the) Gibbs excess mass of and adsorbed in NORIT
R1. In the low pressure region it increases considerably with increasing
pressure to reach a maximum and then falls off but still maintains positive
numerical values. This decrease of the Gibbs excess mass with increasing
pressure in the high pressure region poses a serious thermodynamic problem
as then the stability condition which is a consequence of the
Second Law of Thermodynamics, does not hold, cp. Chaps. 1, 7. As
according to experience adsorbate phases also at high pressures are
thermodynamically stable phases and up to now indications of high pressure
induced phase changes could be observed [3.39, 3.51]. Thus we prefer not to
use the concept of the Gibbs excess mass as a basis for developing
thermodynamics of adsorbate phases. This rather should be done on the basis
of the concept of absolute masses or amounts adsorbed cp. Chaps. 1,
9, the Gibbs excess still being a reasonable approximation for the
absolute mass adsorbed in the low pressure region where the
thermodynamic stability condition mentioned above still holds. Examples for