Page 166 - Gas Adsorption Equilibria
P. 166
152 Chapter 3
the difference between and already have been given in Chap. 1. A
method to measure the absolute mass adsorbed without introducing a
hypothesis on the volume of either the porous solid nor the adsorbate phase is
outlined in Chap. 8, cp. [3.40].
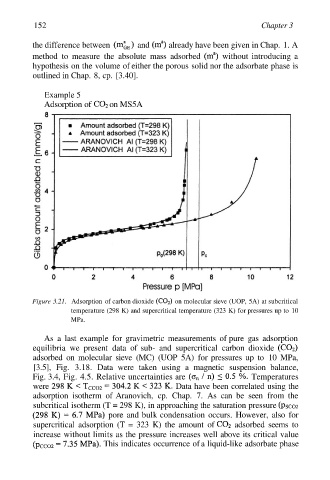
Example 5
Adsorption of on MS5A
Figure 3.21. Adsorption of carbon dioxide on molecular sieve (UOP, 5A) at subcritical
temperature (298 K) and supercritical temperature (323 K) for pressures up to 10
MPa.
As a last example for gravimetric measurements of pure gas adsorption
equilibria we present data of sub- and supercritical carbon dioxide
adsorbed on molecular sieve (MC) (UOP 5A) for pressures up to 10 MPa,
[3.5], Fig. 3.18. Data were taken using a magnetic suspension balance,
Fig. 3.4, Fig. 4.5. Relative uncertainties are Temperatures
were Data have been correlated using the
adsorption isotherm of Aranovich, cp. Chap. 7. As can be seen from the
subcritical isotherm (T = 298 K), in approaching the saturation pressure
pore and bulk condensation occurs. However, also for
supercritical adsorption (T = 323 K) the amount of adsorbed seems to
increase without limits as the pressure increases well above its critical value
This indicates occurrence of a liquid-like adsorbate phase