Page 158 - Gas Adsorption Equilibria
P. 158
144 Chapter 3
Example 3
Adsorption of air compounds and carbon dioxide on zeolite
with presorbed water.
Most zeolites are hydrophilic, i. e. attract water which usually is strongly
adsorbed, i. e. only can be desorbed by heating the zeolite in vacuum or
helium atmosphere up to 700 K. Hence one may conjecture that the amount of
gas adsorbed on a zeolite at ambient temperature will depend on the amount
of water which is permanently presorbed on the zeolite. This is indeed the
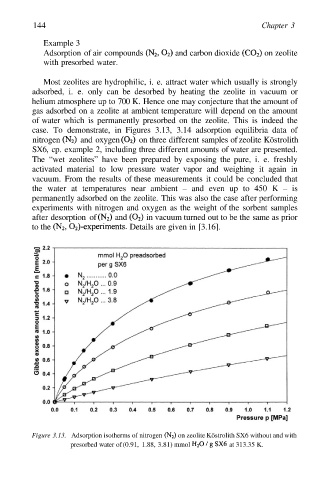
case. To demonstrate, in Figures 3.13, 3.14 adsorption equilibria data of
nitrogen and oxygen on three different samples of zeolite Köstrolith
SX6, cp. example 2, including three different amounts of water are presented.
The “wet zeolites” have been prepared by exposing the pure, i. e. freshly
activated material to low pressure water vapor and weighing it again in
vacuum. From the results of these measurements it could be concluded that
the water at temperatures near ambient – and even up to 450 K – is
permanently adsorbed on the zeolite. This was also the case after performing
experiments with nitrogen and oxygen as the weight of the sorbent samples
after desorption of and in vacuum turned out to be the same as prior
to the Details are given in [3.16].
Figure 3.13. Adsorption isotherms of nitrogen on zeolite Köstrolith SX6 without and with
presorbed water of (0.91, 1.88, 3.81) mmol at 313.35 K.