Page 80 - Geochemical Remote Sensing of The Sub-Surface
P. 80
Geoelectrochemistry and stream dispersion 57
For anodic processes there are also two wave steps on the polarisation curves at
potentials q~ and cp2 corresponding to two electrochemical reactions. The first anodic
electrochemical reaction for sulphide at the potential q~t corresponds to its anodic
decomposition (transition of ions of metals in solution),
MeS --~ Me2++ S~ 2e. (2.27)
The second anodic reaction for sulphides reflects the further oxidation of sulphur. For
example, in the case of galena,
PbS + 4H20 ~ Pb 2+ + 8042--F 8H + + 8e- (2.28)
As a result of these studies on the cathodic and anodic mineral processes, the
electrochemical reaction potentials for the main electron-conducting minerals were
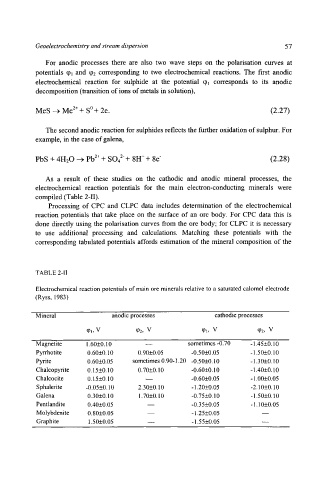
compiled (Table 2-I1).
Processing of CPC and CLPC data includes determination of the electrochemical
reaction potentials that take place on the surface of an ore body. For CPC data this is
done directly using the polarisation curves from the ore body; for CLPC it is necessary
to use additional processing and calculations. Matching these potentials with the
corresponding tabulated potentials affords estimation of the mineral composition of the
TABLE 2-1I
Electrochemical reaction potentials of main ore minerals relative to a saturated calomel electrode
(Ryss, 1983)
Mineral anodic processes cathodic processes
q~l, V qh, V q~l, V tp2, V
Magnetite 1.60+0.10 -- sometimes -0.70 -1.45+0.10
Pyrrhotite 0.60+0.10 0.90+0.05 -0.50+0.05 - 1.50+0.10
Pyrite 0.60+0.05 sometimes 0.90-1.20 -0.50+0.10 -1.30+0.10
Chalcopyrite 0.15+0.10 0.70+0.10 -0.60+0.10 - 1.40+0.10
Chalcocite 0.15+0.10 m -0.60+0.05 - 1.00+0.05
Sphalerite -0.05+0.10 2.30+0.10 - 1.20+0.05 -2.10+0.10
Galena 0.30+0.10 1.70+0.10 -0.75+0.10 -1.50+0.10
Pentland ite 0.40+0.05 m -0.35 +0.05 - 1.10+0.05
Molybdenite 0.80+0.05 - 1.25+0.05
Graphite 1.50+0.05 -- - 1.55+0.05