Page 139 - Geology and Geochemistry of Oil and Gas
P. 139
110 NATURAL GASES AND CONDENSATES
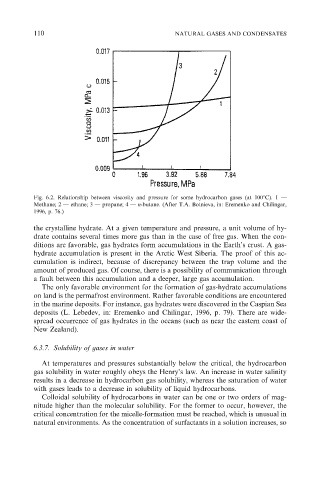
Fig. 6.2. Relationship between viscosity and pressure for some hydrocarbon gases (at 1001C). 1 —
Methane; 2 — ethane; 3 — propane; 4 — n-butane. (After T.A. Botnieva, in: Eremenko and Chilingar,
1996, p. 76.)
the crystalline hydrate. At a given temperature and pressure, a unit volume of hy-
drate contains several times more gas than in the case of free gas. When the con-
ditions are favorable, gas hydrates form accumulations in the Earth’s crust. A gas-
hydrate accumulation is present in the Arctic West Siberia. The proof of this ac-
cumulation is indirect, because of discrepancy between the trap volume and the
amount of produced gas. Of course, there is a possibility of communication through
a fault between this accumulation and a deeper, large gas accumulation.
The only favorable environment for the formation of gas-hydrate accumulations
on land is the permafrost environment. Rather favorable conditions are encountered
in the marine deposits. For instance, gas hydrates were discovered in the Caspian Sea
deposits (L. Lebedev, in: Eremenko and Chilingar, 1996, p. 79). There are wide-
spread occurrence of gas hydrates in the oceans (such as near the eastern coast of
New Zealand).
6.3.7. Solubility of gases in water
At temperatures and pressures substantially below the critical, the hydrocarbon
gas solubility in water roughly obeys the Henry’s law. An increase in water salinity
results in a decrease in hydrocarbon gas solubility, whereas the saturation of water
with gases leads to a decrease in solubility of liquid hydrocarbons.
Colloidal solubility of hydrocarbons in water can be one or two orders of mag-
nitude higher than the molecular solubility. For the former to occur, however, the
critical concentration for the micelle-formation must be reached, which is unusual in
natural environments. As the concentration of surfactants in a solution increases, so