Page 142 - Geology and Geochemistry of Oil and Gas
P. 142
PHASE TRANSFORMATION AND CONDENSATES 113
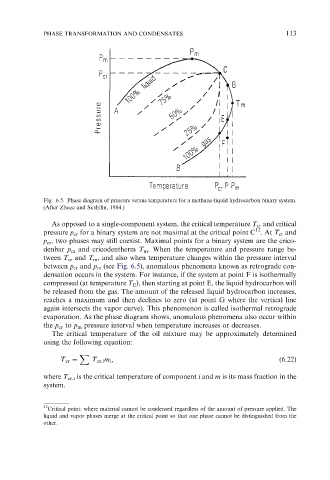
Fig. 6.5. Phase diagram of pressure versus temperature for a methane-liquid hydrocarbon binary system.
(After Zhuze and Sushilin, 1984.)
As opposed to a single-component system, the critical temperature T cr and critical
12
pressure p cr for a binary system are not maximal at the critical point C . At T cr and
p cr , two phases may still coexist. Maximal points for a binary system are the crico-
denbar p m and cricodentherm T m . When the temperature and pressure range be-
tween T cr and T m , and also when temperature changes within the pressure interval
between p cr and p m (see Fig. 6.5), anomalous phenomena known as retrograde con-
densation occurs in the system. For instance, if the system at point F is isothermally
compressed (at temperature T E ), then starting at point E, the liquid hydrocarbon will
be released from the gas. The amount of the released liquid hydrocarbon increases,
reaches a maximum and then declines to zero (at point G where the vertical line
again intersects the vapor curve). This phenomenon is called isothermal retrograde
evaporation. As the phase diagram shows, anomalous phenomena also occur within
the p cr to p m pressure interval when temperature increases or decreases.
The critical temperature of the oil mixture may be approximately determined
using the following equation:
X
T cr ¼ T cr:i m i , (6.22)
where T cr.i is the critical temperature of component i and m is its mass fraction in the
system.
12
Critical point: where material cannot be condensed regardless of the amount of pressure applied. The
liquid and vapor phases merge at the critical point so that one phase cannot be distinguished from the
other.