Page 144 - Geology and Geochemistry of Oil and Gas
P. 144
PHASE TRANSFORMATION AND CONDENSATES 115
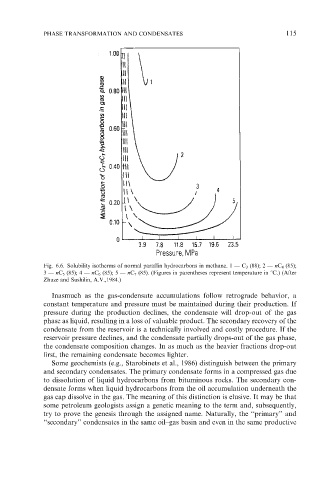
Fig. 6.6. Solubility isotherms of normal paraffin hydrocarbons in methane. 1 — C 3 (88); 2 — nC 4 (85);
3 — nC 5 (85); 4 — nC 6 (85); 5 — nC 7 (85). (Figures in parentheses represent temperature in 1C.) (After
Zhuze and Sushilin, A.V.,1984.)
Inasmuch as the gas-condensate accumulations follow retrograde behavior, a
constant temperature and pressure must be maintained during their production. If
pressure during the production declines, the condensate will drop-out of the gas
phase as liquid, resulting in a loss of valuable product. The secondary recovery of the
condensate from the reservoir is a technically involved and costly procedure. If the
reservoir pressure declines, and the condensate partially drops-out of the gas phase,
the condensate composition changes. In as much as the heavier fractions drop-out
first, the remaining condensate becomes lighter.
Some geochemists (e.g., Starobinets et al., 1986) distinguish between the primary
and secondary condensates. The primary condensate forms in a compressed gas due
to dissolution of liquid hydrocarbons from bituminous rocks. The secondary con-
densate forms when liquid hydrocarbons from the oil accumulation underneath the
gas cap dissolve in the gas. The meaning of this distinction is elusive. It may be that
some petroleum geologists assign a genetic meaning to the term and, subsequently,
try to prove the genesis through the assigned name. Naturally, the ‘‘primary’’ and
‘‘secondary’’ condensates in the same oil–gas basin and even in the same productive