Page 140 - Geology and Geochemistry of Oil and Gas
P. 140
PHYSICAL PROPERTIES OF NATURAL GASES 111
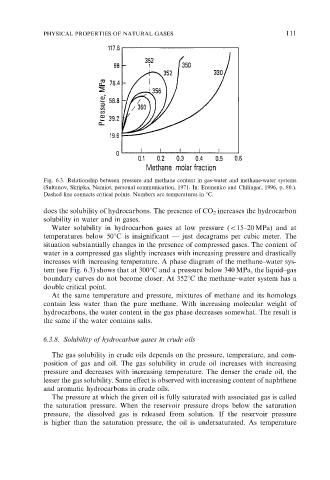
Fig. 6.3. Relationship between pressure and methane content in gas-water and methane-water systems
(Sultanov, Skripka, Namiot, personal communication, 1971. In: Eremenko and Chilingar, 1996, p. 80.).
Dashed line connects critical points. Numbers are temperatures in 1C.
does the solubility of hydrocarbons. The presence of CO 2 increases the hydrocarbon
solubility in water and in gases.
Water solubility in hydrocarbon gases at low pressure (o15–20 MPa) and at
temperatures below 501C is insignificant — just decagrams per cubic meter. The
situation substantially changes in the presence of compressed gases. The content of
water in a compressed gas slightly increases with increasing pressure and drastically
increases with increasing temperature. A phase diagram of the methane–water sys-
tem (see Fig. 6.3) shows that at 3001C and a pressure below 340 MPa, the liquid–gas
boundary curves do not become closer. At 3521C the methane–water system has a
double critical point.
At the same temperature and pressure, mixtures of methane and its homologs
contain less water than the pure methane. With increasing molecular weight of
hydrocarbons, the water content in the gas phase decreases somewhat. The result is
the same if the water contains salts.
6.3.8. Solubility of hydrocarbon gases in crude oils
The gas solubility in crude oils depends on the pressure, temperature, and com-
position of gas and oil. The gas solubility in crude oil increases with increasing
pressure and decreases with increasing temperature. The denser the crude oil, the
lesser the gas solubility. Same effect is observed with increasing content of naphthene
and aromatic hydrocarbons in crude oils.
The pressure at which the given oil is fully saturated with associated gas is called
the saturation pressure. When the reservoir pressure drops below the saturation
pressure, the dissolved gas is released from solution. If the reservoir pressure
is higher than the saturation pressure, the oil is undersaturated. As temperature