Page 40 - Handbook of Adhesion Promoters
P. 40
2.10 Hydrogen bonding 33
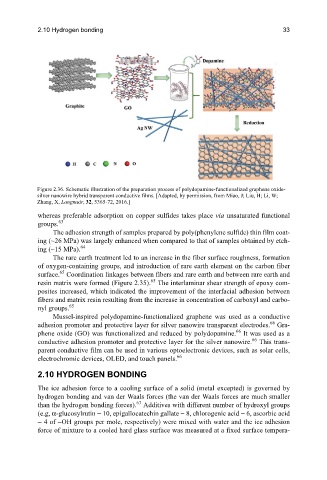
Figure 2.36. Schematic illustration of the preparation process of polydopamine-functionalized graphene oxide-
silver nanowire hybrid transparent conductive films. [Adapted, by permission, from Miao, J; Liu, H; Li, W;
Zhang, X, Langmuir, 32, 5365-72, 2016.]
whereas preferable adsorption on copper sulfides takes place via unsaturated functional
63
groups.
The adhesion strength of samples prepared by poly(phenylene sulfide) thin film coat-
ing (~26 MPa) was largely enhanced when compared to that of samples obtained by etch-
64
ing (~15 MPa).
The rare earth treatment led to an increase in the fiber surface roughness, formation
of oxygen-containing groups, and introduction of rare earth element on the carbon fiber
65
surface. Coordination linkages between fibers and rare earth and between rare earth and
65
resin matrix were formed (Figure 2.35). The interlaminar shear strength of epoxy com-
posites increased, which indicated the improvement of the interfacial adhesion between
fibers and matrix resin resulting from the increase in concentration of carboxyl and carbo-
65
nyl groups.
Mussel-inspired polydopamine-functionalized graphene was used as a conductive
66
adhesion promoter and protective layer for silver nanowire transparent electrodes. Gra-
66
phene oxide (GO) was functionalized and reduced by polydopamine. It was used as a
66
conductive adhesion promoter and protective layer for the silver nanowire. This trans-
parent conductive film can be used in various optoelectronic devices, such as solar cells,
66
electrochromic devices, OLED, and touch panels.
2.10 HYDROGEN BONDING
The ice adhesion force to a cooling surface of a solid (metal excepted) is governed by
hydrogen bonding and van der Waals forces (the van der Waals forces are much smaller
67
than the hydrogen bonding forces). Additives with different number of hydroxyl groups
(e.g, α-glucosylrutin − 10, epigallocatechin gallate − 8, chlorogenic acid − 6, ascorbic acid
− 4 of −OH groups per mole, respectively) were mixed with water and the ice adhesion
force of mixture to a cooled hard glass surface was measured at a fixed surface tempera-