Page 38 - Handbook of Adhesion Promoters
P. 38
2.9 Chemical bonding 31
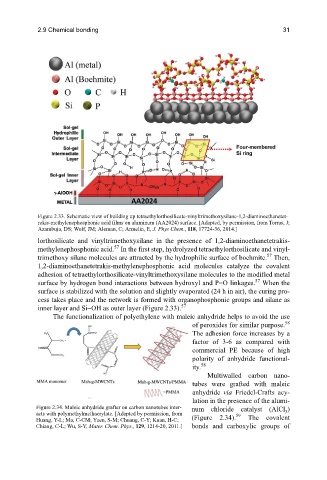
Figure 2.33. Schematic view of building up tetraethylorthosilicate-vinyltrimethoxysilane-1,2-diaminoethanetet-
rakis-methylenephosphonic acid films on aluminum (AA2024) surface. [Adapted, by permission, from Torras, J;
Azambuja, DS; Wolf, JM; Aleman, C; Armelin, E, J. Phys Chem., 118, 17724-36, 2014.]
lorthosilicate and vinyltrimethoxysilane in the presence of 1,2-diaminoethanetetrakis-
57
methylenephosphonic acid. In the first step, hydrolyzed tetraethylorthosilicate and vinyl-
57
trimethoxy silane molecules are attracted by the hydrophilic surface of boehmite. Then,
1,2-diaminoethanetetrakis-methylenephosphonic acid molecules catalyze the covalent
adhesion of tetraethylorthosilicate-vinyltrimethoxysilane molecules to the modified metal
57
surface by hydrogen bond interactions between hydroxyl and P=O linkages. When the
surface is stabilized with the solution and slightly evaporated (24 h in air), the curing pro-
cess takes place and the network is formed with organophosphonic groups and silane as
57
inner layer and Si−OH as outer layer (Figure 2.33).
The functionalization of polyethylene with maleic anhydride helps to avoid the use
of peroxides for similar purpose. 58
The adhesion force increases by a
factor of 3-6 as compared with
commercial PE because of high
polarity of anhydride functional-
58
ity.
Multiwalled carbon nano-
tubes were grafted with maleic
anhydride via Friedel-Crafts acy-
lation in the presence of the alumi-
Figure 2.34. Maleic anhydride grafter on carbon nanotubes inter- num chloride catalyst (AlCl )
3
acts with polymethylmethacrylate. [Adapted by permission, from (Figure 2.34). The covalent
59
Huang, Y-L; Ma, C-CM; Yuen, S-M; Chuang, C-Y; Kuan, H-C;
Chiang, C-L; Wu, S-Y, Mater. Chem. Phys., 129, 1214-20, 2011.] bonds and carboxylic groups of