Page 43 - Handbook of Adhesion Promoters
P. 43
36 Mechanisms of Adhesion
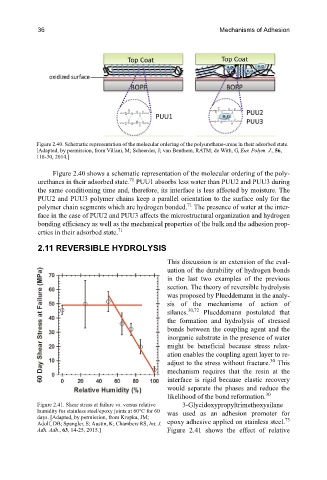
Figure 2.40. Schematic representation of the molecular ordering of the polyurethane-ureas in their adsorbed state.
[Adapted, by permission, from Villani, M; Scheerder, J; van Benthem, RATM; de With, G, Eur. Polym. J., 56,
118-30, 2014.]
Figure 2.40 shows a schematic representation of the molecular ordering of the poly-
71
urethanes in their adsorbed state. PUU1 absorbs less water than PUU2 and PUU3 during
the same conditioning time and, therefore, its interface is less affected by moisture. The
PUU2 and PUU3 polymer chains keep a parallel orientation to the surface only for the
71
polymer chain segments which are hydrogen bonded. The presence of water at the inter-
face in the case of PUU2 and PUU3 affects the microstructural organization and hydrogen
bonding efficiency as well as the mechanical properties of the bulk and the adhesion prop-
71
erties in their adsorbed state.
2.11 REVERSIBLE HYDROLYSIS
This discussion is an extension of the eval-
uation of the durability of hydrogen bonds
in the last two examples of the previous
section. The theory of reversible hydrolysis
was proposed by Plueddemann in the analy-
sis of the mechanisms of action of
silanes. 30,72 Plueddemann postulated that
the formation and hydrolysis of stressed
bonds between the coupling agent and the
inorganic substrate in the presence of water
might be beneficial because stress relax-
ation enables the coupling agent layer to re-
30
adjust to the stress without fracture. This
mechanism requires that the resin at the
interface is rigid because elastic recovery
would separate the phases and reduce the
30
likelihood of the bond reformation.
Figure 2.41. Shear stress at failure vs. versus relative 3-Glycidoxypropyltrimethoxysilane
humidity for stainless steel/epoxy joints at 60°C for 60 was used as an adhesion promoter for
days. [Adapted, by permission, from Kropka, JM; 73
Adolf, DB; Spangler, S; Austin, K; Chambers RS, Int. J. epoxy adhesive applied on stainless steel.
Adh. Adh., 63, 14-25, 2015.] Figure 2.41 shows the effect of relative