Page 57 - Handbook of Adhesion Promoters
P. 57
50 Mechanisms of Adhesion Loss
fraction of debonded particles increases because of an increase in the overlap of the stress
17
fields caused by a reduced average interparticle separation.
3.5 LIQUID PENETRATION
This section includes continua-
tion of the discussion of delamina-
tion caused by the effect of water
with a focus on the effects of
moisture concentration at the
interface. In the earlier studies of
adhesion loss due to the action of
environmental factors, the funda-
mental mechanisms of adhesion
loss at a critical relative humidity
was studied for polymethyl-
methacrylate by combining the
detailed characterization of the
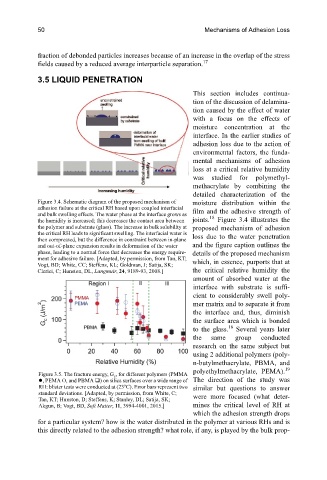
Figure 3.4. Schematic diagram of the proposed mechanism of moisture distribution within the
adhesion failure at the critical RH based upon coupled interfacial film and the adhesive strength of
and bulk swelling effects. The water phase at the interface grows as 18
the humidity is increased; this decreases the contact area between joints. Figure 3.4 illustrates the
the polymer and substrate (glass). The increase in bulk solubility at proposed mechanism of adhesion
the critical RH leads to significant swelling. The interfacial water is loss due to the water penetration
then compressed, but the difference in constraint between in-plane
and out-of-plane expansion results in deformation of the water and the figure caption outlines the
phase, leading to a normal force that decreases the energy require- details of the proposed mechanism
ment for adhesive failure. [Adapted, by permission, from Tan, KT; which, in essence, purports that at
Vogt, BD; White, CC; Steffens, KL; Goldman, J; Satija, SK;
Clerici, C; Hunston, DL, Langmuir, 24, 9189-93, 2008.] the critical relative humidity the
amount of absorbed water at the
interface with substrate is suffi-
cient to considerably swell poly-
mer matrix and to separate it from
the interface and, thus, diminish
the surface area which is bonded
18
to the glass. Several years later
the same group conducted
research on the same subject but
using 2 additional polymers (poly-
n-butylmethacrylate, PBMA, and
polyethylmethacrylate, PEMA). 19
Figure 3.5. The fracture energy, G c , for different polymers (PMMA
, PEMA O, and PBMA ) on silica surfaces over a wide range of The direction of the study was
o
RH: blister tests were conducted at (23 C). Error bars represent two similar but questions to answer
standard deviations. [Adapted, by permission, from White, C; were more focused (what deter-
Tan, KT; Hunston, D; Steffens, K; Stanley, DL; Satija, SK;
Akgun, B; Vogt, BD, Soft Matter, 11, 3994-4001, 2015.] mines the critical level of RH at
which the adhesion strength drops
for a particular system? how is the water distributed in the polymer at various RHs and is
this directly related to the adhesion strength? what role, if any, is played by the bulk prop-