Page 109 - Handbook of Battery Materials
P. 109
2.7 Lithium-Ion Batteries 75
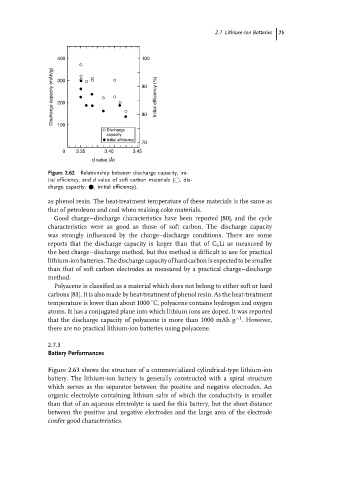
400 100
Discharge capacity (mAh/g) 300 90 Initial efficiency (%)
200
100
Discharge 80
capacity
Initial efficiency
70
0 3.35 3.40 3.45
d value (Å)
Figure 2.62 Relationship between discharge capacity, ini-
tial efficiency, and d value of soft carbon materials ( ,dis-
charge capacity; • , initial efficiency).
as phenol resin. The heat-treatment temperature of these materials is the same as
that of petroleum and coal when making coke materials.
Good charge–discharge characteristics have been reported [80], and the cycle
characteristics were as good as those of soft carbon. The discharge capacity
was strongly influenced by the charge–discharge conditions. There are some
reports that the discharge capacity is larger than that of C 6 Li as measured by
the best charge–discharge method, but this method is difficult to use for practical
lithium-ion batteries. The discharge capacity of hard carbon is expected to be smaller
than that of soft carbon electrodes as measured by a practical charge–discharge
method.
Polyacene is classified as a material which does not belong to either soft or hard
carbons [81]. It is also made by heat-treatment of phenol resin. As the heat-treatment
◦
temperature is lower than about 1000 C, polyacene contains hydrogen and oxygen
atoms. It has a conjugated plane into which lithium ions are doped. It was reported
−1
that the discharge capacity of polyacene is more than 1000 mAh g . However,
there are no practical lithium-ion batteries using polyacene.
2.7.3
Battery Performances
Figure 2.63 shows the structure of a commercialized cylindrical-type lithium-ion
battery. The lithium-ion battery is generally constructed with a spiral structure
which serves as the separator between the positive and negative electrodes. An
organic electrolyte containing lithium salts of which the conductivity is smaller
than that of an aqueous electrolyte is used for this battery, but the short distance
between the positive and negative electrodes and the large area of the electrode
confer good characteristics.