Page 107 - Handbook of Battery Materials
P. 107
2.7 Lithium-Ion Batteries 73
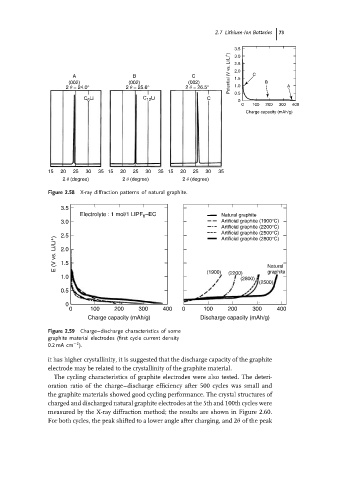
3.5
Potential (V vs. Li/Li + ) 2.5 C
3.0
2.0
A B C 1.5
(002) (002) (002) B
2 q = 24.0° 2 q = 25.6° 2 q = 26.5° 1.0 A
C Li C 12 Li C 0.5 0
6
0 100 200 300 400
Charge capacity (mAh/g)
15 20 25 30 35 15 20 25 30 35 15 20 25 30 35
2 q (degree) 2 q (degree) 2 q (degree)
Figure 2.58 X-ray diffraction patterns of natural graphite.
3.5
Electrolyte : 1 mol/1 LIPF 6 –EC Natural graphite
3.0 Artificial graphite (1900°C)
Artificial graphite (2200°C)
Artificial graphite (2500°C)
2.5 Artificial graphite (2800°C)
E (V vs. Li/Li + ) 2.0
1.5
graphite
1.0 (1900) (2200) Natural
(2800)
(2500)
0.5
0
0 100 200 300 400 0 100 200 300 400
Charge capacity (mAh/g) Discharge capacity (mAh/g)
Figure 2.59 Charge–discharge characteristics of some
graphite material electrodes (first cycle current density
−2
0.2 mA cm ).
it has higher crystallinity, it is suggested that the discharge capacity of the graphite
electrode may be related to the crystallinity of the graphite material.
The cycling characteristics of graphite electrodes were also tested. The deteri-
oration ratio of the charge–discharge efficiency after 500 cycles was small and
the graphite materials showed good cycling performance. The crystal structures of
charged and discharged natural graphite electrodes at the 5th and 100th cycles were
measured by the X-ray diffraction method; the results are shown in Figure 2.60.
For both cycles, the peak shifted to a lower angle after charging, and 2θ of the peak