Page 108 - Handbook of Battery Materials
P. 108
74 2 Practical Batteries
after discharging
after charging
2 q = 24° 2 q = 24°
20 30 20 30
2 q (degree) 2 q (degree)
at 5th cycle at 100th cycle
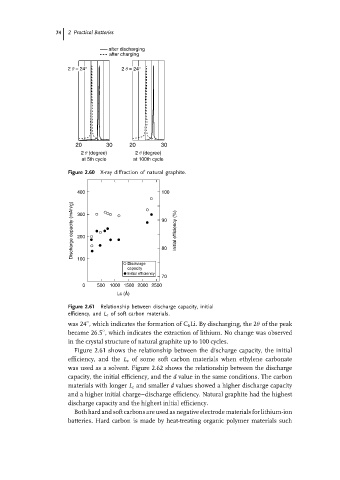
Figure 2.60 X-ray diffraction of natural graphite.
400 100
Discharge capacity (mAh/g) 300 90 Initial efficiency (%)
200
100
Discharge 80
capacity
Initial efficiency
70
0 500 1000 1500 2000 2500
Lc (Å)
Figure 2.61 Relationship between discharge capacity, initial
efficiency, and L c of soft carbon materials.
◦
was 24 , which indicates the formation of C 6 Li. By discharging, the 2θ of the peak
◦
became 26.5 , which indicates the extraction of lithium. No change was observed
in the crystal structure of natural graphite up to 100 cycles.
Figure 2.61 shows the relationship between the discharge capacity, the initial
efficiency, and the L c of some soft carbon materials when ethylene carbonate
was used as a solvent. Figure 2.62 shows the relationship between the discharge
capacity, the initial efficiency, and the d value in the same conditions. The carbon
materials with longer L c and smaller d values showed a higher discharge capacity
and a higher initial charge–discharge efficiency. Natural graphite had the highest
discharge capacity and the highest initial efficiency.
Bothhard and soft carbons are used as negative electrode materials for lithium-ion
batteries. Hard carbon is made by heat-treating organic polymer materials such