Page 103 - Handbook of Battery Materials
P. 103
2.7 Lithium-Ion Batteries 69
4.5
LiCoO 2 LiCoO 2
(850°C Air) (850°C O )
2
4.0
E (V vs. Li/Li + ) 3.5 LiNiO (750°C Air)
2
3.0
LiNiO 2 (750°C O )
2
2.5
0 50 100 150 200
Discharge capacity (mAh/g)
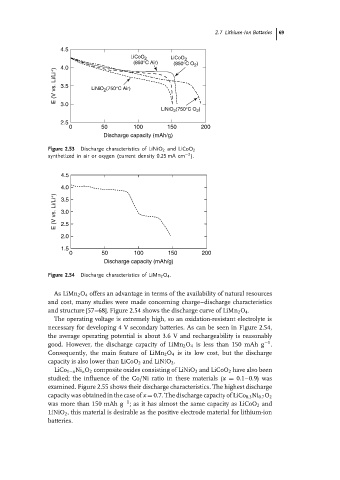
Figure 2.53 Discharge characteristics of LiNiO 2 and LiCoO 2
−2
synthetized in air or oxygen (current density 0.25 mA cm ).
4.5
4.0
E (V vs. Li/Li + ) 3.0
3.5
2.5
2.0
1.5
0 50 100 150 200
Discharge capacity (mAh/g)
Figure 2.54 Discharge characteristics of LiMn 2 O 4 .
As LiMn 2 O 4 offers an advantage in terms of the availability of natural resources
and cost, many studies were made concerning charge–discharge characteristics
and structure [57–68]. Figure 2.54 shows the discharge curve of LiMn 2 O 4 .
The operating voltage is extremely high, so an oxidation-resistant electrolyte is
necessary for developing 4 V secondary batteries. As can be seen in Figure 2.54,
the average operating potential is about 3.6 V and rechargeability is reasonably
−1
good. However, the discharge capacity of LiMn 2 O 4 is less than 150 mAh g .
Consequently, the main feature of LiMn 2 O 4 is its low cost, but the discharge
capacity is also lower than LiCoO 2 and LiNiO 2 .
LiCo 1−x Ni x O 2 composite oxides consisting of LiNiO 2 and LiCoO 2 have also been
studied; the influence of the Co/Ni ratio in these materials (x = 0.1–0.9) was
examined. Figure 2.55 shows their discharge characteristics. The highest discharge
capacity was obtained in the case of x = 0.7. The discharge capacity of LiCo 0.3 Ni 0.7 O 2
−1
was more than 150 mAh g ; as it has almost the same capacity as LiCoO 2 and
LiNiO 2 , this material is desirable as the positive electrode material for lithium-ion
batteries.