Page 104 - Handbook of Battery Materials
P. 104
70 2 Practical Batteries
5 4 LiCo 0.3 Ni 0.7 O 2
Potential (V vs. Li/Li + ) 3 2 LiCo 0.9 Ni 0.1 O 2 0.2 2 LiCo 0.1 Ni 0.9 O 2 LiCo LiCo 0.7 Ni 0.3 O 2
Ni
O
0.5 2
0.5
LiCo
Ni
O
0.8
LiCo 0.2 Ni 0.8 O 2
LiCo 0.4 Ni 0.6 O 2
0 1 LiCo 0.6 Ni 0.4 O 2
0 50 100 150 200
Discharge capacity (mAh/g)
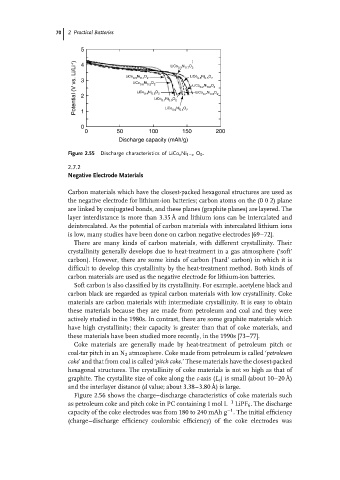
Figure 2.55 Discharge characteristics of LiCo x Ni 1−x O 0 .
2.7.2
Negative Electrode Materials
Carbon materials which have the closest-packed hexagonal structures are used as
the negative electrode for lithium-ion batteries; carbon atoms on the (0 0 2) plane
are linked by conjugated bonds, and these planes (graphite planes) are layered. The
layer interdistance is more than 3.35 ˚ A and lithium ions can be intercalated and
deintercalated. As the potential of carbon materials with intercalated lithium ions
is low, many studies have been done on carbon negative electrodes [69–72].
There are many kinds of carbon materials, with different crystallinity. Their
crystallinity generally develops due to heat-treatment in a gas atmosphere (‘soft’
carbon). However, there are some kinds of carbon (‘hard’ carbon) in which it is
difficult to develop this crystallinity by the heat-treatment method. Both kinds of
carbon materials are used as the negative electrode for lithium-ion batteries.
Soft carbon is also classified by its crystallinity. For example, acetylene black and
carbon black are regarded as typical carbon materials with low crystallinity. Coke
materials are carbon materials with intermediate crystallinity. It is easy to obtain
these materials because they are made from petroleum and coal and they were
actively studied in the 1980s. In contrast, there are some graphite materials which
have high crystallinity; their capacity is greater than that of coke materials, and
these materials have been studied more recently, in the 1990s [73–77].
Coke materials are generally made by heat-treatment of petroleum pitch or
coal-tar pitch in an N 2 atmosphere. Coke made from petroleum is called ‘petroleum
coke’ and that from coal is called ‘pitch coke.’ These materials have the closest-packed
hexagonal structures. The crystallinity of coke materials is not so high as that of
graphite. The crystallite size of coke along the c-axis (L c ) is small (about 10–20 ˚ A)
and the interlayer distance (d value; about 3.38–3.80 ˚ A) is large.
Figure 2.56 shows the charge–discharge characteristics of coke materials such
as petroleum coke and pitch coke in PC containing 1 mol L −1 LiPF 6 . The discharge
−1
capacity of the coke electrodes was from 180 to 240 mAh g . The initial efficiency
(charge–discharge efficiency coulombic efficiency) of the coke electrodes was