Page 101 - Handbook of Battery Materials
P. 101
2.7 Lithium-Ion Batteries 67
4.5
Heat treatment : 850°C
4.0 LiCO 2
E (V vs. Li/Li + ) 3.5 850°C)
(Li 2 CO 2 + CoCO 2
3.0
2.5
0 50 100 150 200
Discharge capacity (mAh/g)
−2
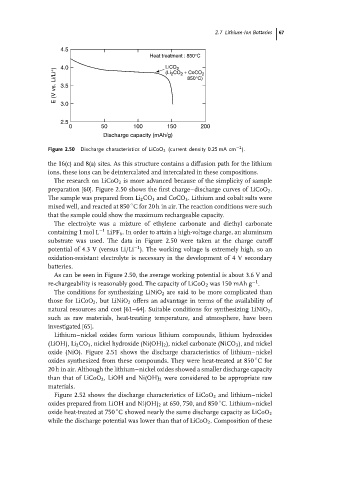
Figure 2.50 Discharge characteristics of LiCoO 2 (current density 0.25 mA cm ).
the 16(c) and 8(a) sites. As this structure contains a diffusion path for the lithium
ions, these ions can be deintercalated and intercalated in these compositions.
The research on LiCoO 2 is more advanced because of the simplicity of sample
preparation [60]. Figure 2.50 shows the first charge–discharge curves of LiCoO 2 .
The sample was prepared from Li 2 CO 3 and CoCO 3 . Lithium and cobalt salts were
◦
mixed well, and reacted at 850 C for 20 h in air. The reaction conditions were such
that the sample could show the maximum rechargeable capacity.
The electrolyte was a mixture of ethylene carbonate and diethyl carbonate
containing 1 mol L −1 LiPF 6 . In order to attain a high-voltage charge, an aluminum
substrate was used. The data in Figure 2.50 were taken at the charge cutoff
−1
potential of 4.3 V (versus Li/Li ). The working voltage is extremely high, so an
oxidation-resistant electrolyte is necessary in the development of 4 V secondary
batteries.
As can be seen in Figure 2.50, the average working potential is about 3.6 V and
−1
re-chargeability is reasonably good. The capacity of LiCoO 2 was 150 mAh g .
The conditions for synthesizing LiNiO 2 are said to be more complicated than
those for LiCoO 2 , but LiNiO 2 offers an advantage in terms of the availability of
natural resources and cost [61–64]. Suitable conditions for synthesizing LiNiO 2 ,
such as raw materials, heat-treating temperature, and atmosphere, have been
investigated [65].
Lithium–nickel oxides form various lithium compounds, lithium hydroxides
(LiOH), Li 2 CO 3 , nickel hydroxide (Ni(OH) 2 ), nickel carbonate (NiCO 3 ), and nickel
oxide (NiO). Figure 2.51 shows the discharge characteristics of lithium–nickel
◦
oxides synthesized from these compounds. They were heat-treated at 850 C for
20 h in air. Although the lithium–nickel oxides showed a smaller discharge capacity
than that of LiCoO 2 , LiOH and Ni(OH) 2 were considered to be appropriate raw
materials.
Figure 2.52 shows the discharge characteristics of LiCoO 2 and lithium–nickel
◦
oxides prepared from LiOH and Ni(OH) 2 at 650, 750, and 850 C. Lithium–nickel
◦
oxide heat-treated at 750 C showed nearly the same discharge capacity as LiCoO 2
while the discharge potential was lower than that of LiCoO 2 . Composition of these