Page 96 - Handbook of Battery Materials
P. 96
62 2 Practical Batteries
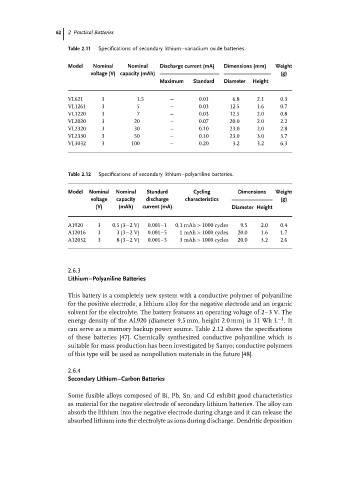
Table 2.11 Specifications of secondary lithium–vanadium oxide batteries.
Model Nominal Nominal Discharge current (mA) Dimensions (mm) Weight
voltage (V) capacity (mAh) (g)
Maximum Standard Diameter Height
VL621 3 1.5 – 0.01 6.8 2.1 0.3
VL1261 3 5 – 0.03 12.5 1.6 0.7
VL1220 3 7 – 0.03 12.5 2.0 0.8
VL2020 3 20 – 0.07 20.0 2.0 2.2
VL2320 3 30 – 0.10 23.0 2.0 2.8
VL2330 3 50 – 0.10 23.0 3.0 3.7
VL3032 3 100 – 0.20 3.2 3.2 6.3
Table 2.12 Specifications of secondary lithium–polyaniline batteries.
Model Nominal Nominal Standard Cycling Dimensions Weight
voltage capacity discharge characteristics (g)
(V) (mAh) current (mA) Diameter Height
A1920 3 0.5 (3−2 V) 0.001–1 0.1 mAh > 1000 cycles 9.5 2.0 0.4
A12016 3 3 (3−2 V) 0.001–5 1 mAh > 1000 cycles 20.0 1.6 1.7
A12032 3 8 (3−2 V) 0.001–5 3 mAh > 1000 cycles 20.0 3.2 2.6
2.6.3
Lithium–Polyaniline Batteries
This battery is a completely new system with a conductive polymer of polyaniline
for the positive electrode, a lithium alloy for the negative electrode and an organic
solvent for the electrolyte. The battery features an operating voltage of 2–3 V. The
−1
energy density of the AL920 (diameter 9.5 mm, height 2.0 mm) is 11 Wh L .It
can serve as a memory backup power source. Table 2.12 shows the specifications
of these batteries [47]. Chemically synthesized conductive polyaniline which is
suitable for mass production has been investigated by Sanyo; conductive polymers
of this type will be used as nonpollution materials in the future [48].
2.6.4
Secondary Lithium–Carbon Batteries
Some fusible alloys composed of Bi, Pb, Sn, and Cd exhibit good characteristics
as material for the negative electrode of secondary lithium batteries. The alloy can
absorb the lithium into the negative electrode during charge and it can release the
absorbed lithium into the electrolyte as ions during discharge. Dendritic deposition