Page 91 - Handbook of Battery Materials
P. 91
2.6 Coin-Type Lithium Secondary Batteries 57
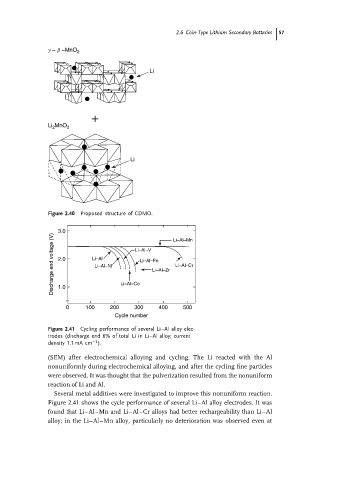
g − b −MnO 2
Li
Li MnO 3
2
Li
Figure 2.40 Proposed structure of CDMO.
3.0 Li–Al–Mn
Discharge end voltage (V) 2.0 Li–Al Li–Al–V Li–Al–Zr Li–Al–Cr
Li–Al–Fe
Li–Al–Nl
1.0
≈ Li–Al–Co
0 100 200 300 400 500
Cycle number
Figure 2.41 Cycling performance of several Li–Al alloy elec-
trodes (discharge end 6% of total Li in Li–Al alloy; current
−2
density 1.1 mA cm ).
(SEM) after electrochemical alloying and cycling. The Li reacted with the Al
nonuniformly during electrochemical alloying, and after the cycling fine particles
were observed. It was thought that the pulverization resulted from the nonuniform
reaction of Li and Al.
Several metal additives were investigated to improve this nonuniform reaction.
Figure 2.41 shows the cycle performance of several Li–Al alloy electrodes. It was
found that Li–Al–Mn and Li–Al–Cr alloys had better rechargeability than Li–Al
alloy; in the Li–Al–Mn alloy, particularly no deterioration was observed even at