Page 94 - Handbook of Battery Materials
P. 94
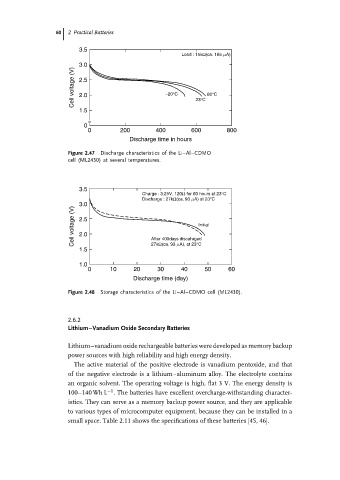
60 2 Practical Batteries
3.5
Load : 15kΩ(ca. 165 mA)
3.0
Cell voltage (V) 2.5 −20°C 60°C
2.0
1.5 23°C
≈
0
0 200 400 600 800
Discharge time in hours
Figure 2.47 Discharge characteristics of the Li–Al–CDMO
cell (ML2430) at several temperatures.
3.5
Charge : 3.25V, 120Ω for 60 hours at 23°C
Discharge : 27kΩ(ca. 93 mA) at 23°C
3.0
Cell voltage (V) 2.5 Initial
2.0
27kΩ(ca. 93 mA), at 23°C
1.5 After 400days discahrged
1.0
0 10 20 30 40 50 60
Discharge time (day)
Figure 2.48 Storage characteristics of the Li–Al–CDMO cell (ML2430).
2.6.2
Lithium–Vanadium Oxide Secondary Batteries
Lithium–vanadium oxide rechargeable batteries were developed as memory backup
power sources with high reliability and high energy density.
The active material of the positive electrode is vanadium pentoxide, and that
of the negative electrode is a lithium–aluminum alloy. The electrolyte contains
an organic solvent. The operating voltage is high, flat 3 V. The energy density is
−1
100–140 Wh L . The batteries have excellent overcharge-withstanding character-
istics. They can serve as a memory backup power source, and they are applicable
to various types of microcomputer equipment, because they can be installed in a
small space. Table 2.11 shows the specifications of these batteries [45, 46].