Page 92 - Handbook of Battery Materials
P. 92
58 2 Practical Batteries
the 500th cycle. It was confirmed by SEM that the Li–Al–Mn alloy did not turn to
powder after cycling. Based on these results, Li–Al–Mn alloy was chosen as the
negative electrode material for coin-type secondary lithium batteries.
Figure 2.42 shows the structure of the ML series of secondary lithium-manganese
dioxide batteries, and Figure 2.43 shows the discharge curves of the ML2430 cell
(diameter 24.5 mm, height 3.0 mm). The nominal voltage and capacity of the
−1
ML2430 are 3 V and 90 mAh, respectively, and the energy density is 160 Wh .
Figures 2.44 and 2.45 show the pulse characteristics and the dependence of
discharge capacity on load; the discharge capacity is 90 mAh, even with a 1 k load.
As regards the cycle performance, the ML2430 exhibits 3000 cycles at 5% depth
and 500 cycles at a 20% depth of charge (Figure 2.46). It can be used over a wide
◦
◦
range of temperatures, from −20 to 60 C. The discharge capacity at −20 Cis
◦
90% of the discharge capacity at 23 C (Figure 2.47). The storage characteristics
of the ML2430 were also measured (Figure 2.48); storage for 60 days at 60 Cis
◦
considered to be equivalent to storage for three years at room temperature. The
loss of discharge capacity is less than 5% per year [39–44].
Finally, Table 2.10 shows the specifications of secondary lithium–manganese
dioxide batteries. Recently, the use of these batteries as sources for memory backup
has expanded remarkably [44].
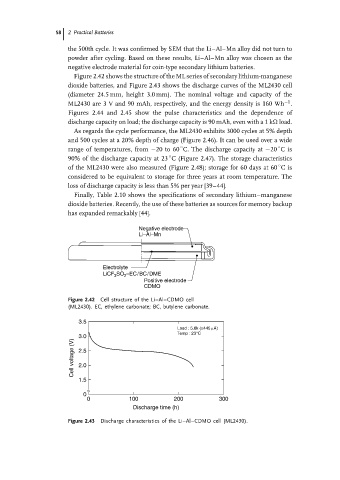
Negative electrode
Li–Al–Mn
Electrolyte
LiCF 3 SO 2 –EC/BC/DME
Positive electrode
CDMO
Figure 2.42 Cell structure of the Li–Al–CDMO cell
(ML2430). EC, ethylene carbonate; BC, butylene carbonate.
3.5
Load : 5.6k (≅445mA)
Temp : 23°C
3.0
Cell voltage (V) 2.5
2.0
1.5
≈
0
0 100 200 300
Discharge time (h)
Figure 2.43 Discharge characteristics of the Li–Al–CDMO cell (ML2430).