Page 90 - Handbook of Battery Materials
P. 90
56 2 Practical Batteries
CDMO
O
spinel LiMn 2 4
4.0 g / b −Mn O
2
Voltage (V) 3.0
2.0
≈
0 0.2 0.4 0.6 0.8 1.0
Li/Mn
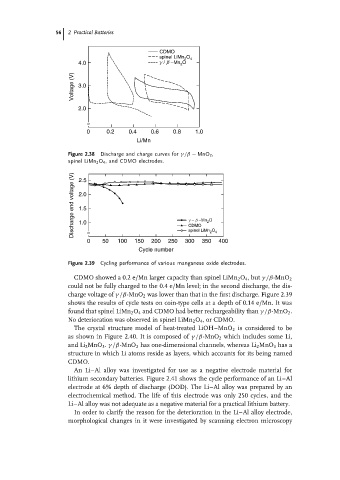
Figure 2.38 Discharge and charge curves for γ/β − MnO 2 ,
spinel LiMn 2 O 4 , and CDMO electrodes.
Discharge end voltage (V) 2.0 g − b −Mn O
2.5
1.5
1.0
2
CDMO
≈
spinel LiMn 2 4
0 50 100 150 200 250 300 350 O 400
Cycle number
Figure 2.39 Cycling performance of various manganese oxide electrodes.
CDMO showed a 0.2 e/Mn larger capacity than spinel LiMn 2 O 4 ,but γ/β-MnO 2
could not be fully charged to the 0.4 e/Mn level; in the second discharge, the dis-
charge voltage of γ/β-MnO 2 was lower than that in the first discharge. Figure 2.39
shows the results of cycle tests on coin-type cells at a depth of 0.14 e/Mn. It was
found that spinel LiMn 2 O 4 and CDMO had better rechargeability than γ/β-MnO 2 .
No deterioration was observed in spinel LiMn 2 O 4 ,orCDMO.
The crystal structure model of heat-treated LiOH–MnO 2 is considered to be
as shown in Figure 2.40. It is composed of γ/β-MnO 2 which includes some Li,
and Li 2 MnO 3 . γ/β-MnO 2 has one-dimensional channels, whereas Li 2 MnO 3 has a
structure in which Li atoms reside as layers, which accounts for its being named
CDMO.
An Li–Al alloy was investigated for use as a negative electrode material for
lithium secondary batteries. Figure 2.41 shows the cycle performance of an Li–Al
electrode at 6% depth of discharge (DOD). The Li–Al alloy was prepared by an
electrochemical method. The life of this electrode was only 250 cycles, and the
Li–Al alloy was not adequate as a negative material for a practical lithium battery.
In order to clarify the reason for the deterioration in the Li–Al alloy electrode,
morphological changes in it were investigated by scanning electron microscopy