Page 105 - Handbook of Battery Materials
P. 105
2.7 Lithium-Ion Batteries 71
3.5
Electrolyte : 1mol/1 LiPF –PC
6
3.0
2.5
E (V vs. Li/Li + ) 2.0 Pitch coke(1400°C)
1.5
1.0
Pitch coke(1200°C)
0.5 Pitch coke(1400°C)
Pitch coke(1200°C)
0
0 50 100 150 200 250 300 350 400 0 50 100 150 200 250
Charge capacity (mAh/g) Discharge capacity (mAh/g)
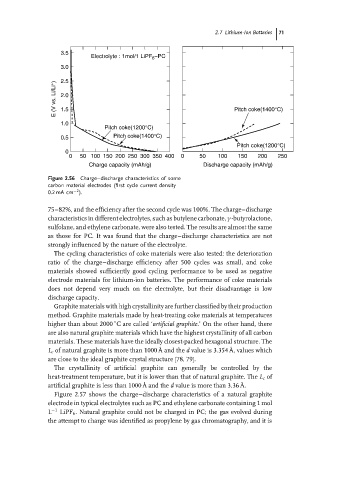
Figure 2.56 Charge–discharge characteristics of some
carbon material electrodes (first cycle current density
−2
0.2 mA cm ).
75–82%, and the efficiency after the second cycle was 100%. The charge–discharge
characteristics in different electrolytes, such as butylene carbonate, γ -butyrolactone,
sulfolane, and ethylene carbonate, were also tested. The results are almost the same
as those for PC. It was found that the charge–discharge characteristics are not
strongly influenced by the nature of the electrolyte.
The cycling characteristics of coke materials were also tested: the deterioration
ratio of the charge–discharge efficiency after 500 cycles was small, and coke
materials showed sufficiently good cycling performance to be used as negative
electrode materials for lithium-ion batteries. The performance of coke materials
does not depend very much on the electrolyte, but their disadvantage is low
discharge capacity.
Graphite materials with high crystallinity are further classified by their production
method. Graphite materials made by heat-treating coke materials at temperatures
◦
higher than about 2000 C are called ‘artificial graphite.’ On the other hand, there
are also natural graphite materials which have the highest crystallinity of all carbon
materials. These materials have the ideally closest-packed hexagonal structure. The
L c of natural graphite is more than 1000 ˚ A and the d value is 3.354 ˚ A, values which
are close to the ideal graphite crystal structure [78, 79].
The crystallinity of artificial graphite can generally be controlled by the
heat-treatment temperature, but it is lower than that of natural graphite. The L c of
artificial graphite is less than 1000 ˚ A and the d value is more than 3.36 ˚ A.
Figure 2.57 shows the charge–discharge characteristics of a natural graphite
electrode in typical electrolytes such as PC and ethylene carbonate containing 1 mol
L −1 LiPF 6 . Natural graphite could not be charged in PC; the gas evolved during
the attempt to charge was identified as propylene by gas chromatography, and it is