Page 106 - Handbook of Battery Materials
P. 106
72 2 Practical Batteries
3.5
3.0
2.5
E (V vs. Li/Li + ) 2.0 EC
1.5
1.0 EC
0.5 PC
0
0 100 200 300 400 0 100 200 300 400
Charge capacity (mAh/g) Discharge capacity (mAh/g)
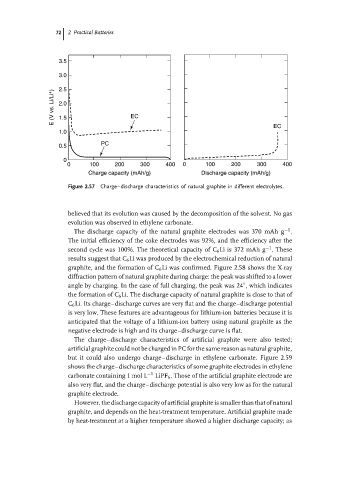
Figure 2.57 Charge–discharge characteristics of natural graphite in different electrolytes.
believed that its evolution was caused by the decomposition of the solvent. No gas
evolution was observed in ethylene carbonate.
−1
The discharge capacity of the natural graphite electrodes was 370 mAh g .
The initial efficiency of the coke electrodes was 92%, and the efficiency after the
−1
second cycle was 100%. The theoretical capacity of C 6 Li is 372 mAh g .These
results suggest that C 6 Li was produced by the electrochemical reduction of natural
graphite, and the formation of C 6 Li was confirmed. Figure 2.58 shows the X-ray
diffraction pattern of natural graphite during charge: the peak was shifted to a lower
◦
angle by charging. In the case of full charging, the peak was 24 , which indicates
the formation of C 6 Li. The discharge capacity of natural graphite is close to that of
C 6 Li. Its charge–discharge curves are very flat and the charge–discharge potential
is very low. These features are advantageous for lithium-ion batteries because it is
anticipated that the voltage of a lithium-ion battery using natural graphite as the
negative electrode is high and its charge–discharge curve is flat.
The charge–discharge characteristics of artificial graphite were also tested;
artificial graphite could not be charged in PC for the same reason as natural graphite,
but it could also undergo charge–discharge in ethylene carbonate. Figure 2.59
shows the charge–discharge characteristics of some graphite electrodes in ethylene
carbonate containing 1 mol L −1 LiPF 6 . Those of the artificial graphite electrode are
also very flat, and the charge–discharge potential is also very low as for the natural
graphite electrode.
However, the discharge capacity of artificial graphite is smaller than that of natural
graphite, and depends on the heat-treatment temperature. Artificial graphite made
by heat-treatment at a higher temperature showed a higher discharge capacity; as