Page 102 - Handbook of Battery Materials
P. 102
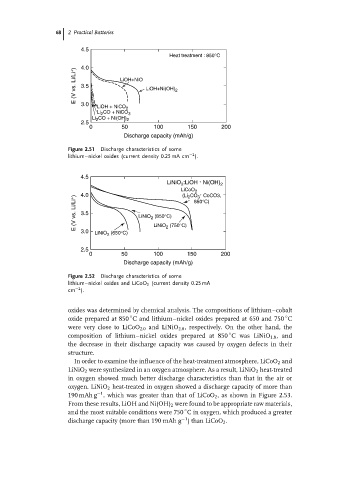
68 2 Practical Batteries
4.5
Heat treatment : 850°C
4.0
E (V vs. Li/Li + ) 3.5 LiOH+NiO LiOH+Ni(OH) 2
3.0
LiOH + NiCO 2
Li CO + NiCO 3
2
Li 2 CO + Ni(OH) 2
2.5
0 50 100 150 200
Discharge capacity (mAh/g)
Figure 2.51 Discharge characteristics of some
−2
lithium–nickel oxides (current density 0.25 mA cm ).
4.5
LiNiO 2 :LiOH Ni(OH) 2
LiCoO 2 850°C)
4.0
(Li CO
CoCO3,
E (V vs. Li/Li + ) 3.5 LiNiO 2 (850°C)
3
2
(750°C)
LiNiO 2
3.0
2.5 LiNiO 2 (650°C)
0 50 100 150 200
Discharge capacity (mAh/g)
Figure 2.52 Discharge characteristics of some
lithium–nickel oxides and LiCoO 2 (current density 0.25 mA
−2
cm ).
oxides was determined by chemical analysis. The compositions of lithium–cobalt
◦
◦
oxide prepared at 850 C and lithium–nickel oxides prepared at 650 and 750 C
were very close to LiCoO 2.0 and LiNiO 2.0 , respectively. On the other hand, the
◦
composition of lithium–nickel oxides prepared at 850 C was LiNiO 1.8 ,and
the decrease in their discharge capacity was caused by oxygen defects in their
structure.
In order to examine the influence of the heat-treatment atmosphere, LiCoO 2 and
LiNiO 2 were synthesized in an oxygen atmosphere. As a result, LiNiO 2 heat-treated
in oxygen showed much better discharge characteristics than that in the air or
oxygen. LiNiO 2 heat-treated in oxygen showed a discharge capacity of more than
−1
190 mAh g , which was greater than that of LiCoO 2 , as shown in Figure 2.53.
From these results, LiOH and Ni(OH) 2 were found to be appropriate raw materials,
◦
and the most suitable conditions were 750 C in oxygen, which produced a greater
−1
discharge capacity (more than 190 mAh g ) than LiCoO 2 .