Page 50 - Handbook of Materials Failure Analysis
P. 50
3 Case Studies 43
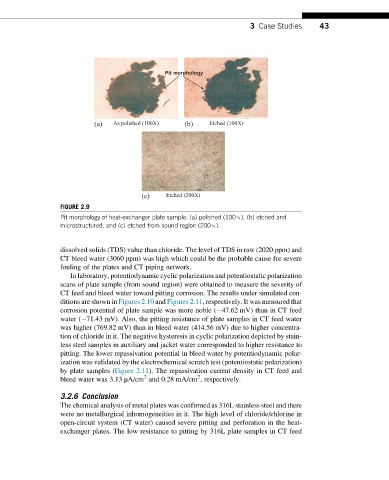
(a) As polished (100X) (b) Etched (100X)
(c) Etched (200X)
FIGURE 2.9
Pit morphology of heat-exchanger plate sample. (a) polished (100 ), (b) etched and
microstructured, and (c) etched from sound region (200 ).
dissolved solids (TDS) value than chloride. The level of TDS in raw (2020 ppm) and
CT bleed water (3060 ppm) was high which could be the probable cause for severe
fouling of the plates and CT piping network.
In laboratory, potentiodynamic cyclic polarization and potentiostatic polarization
scans of plate sample (from sound region) were obtained to measure the severity of
CT feed and bleed water toward pitting corrosion. The results under simulated con-
ditions are shown in Figures 2.10 and Figures 2.11, respectively. It was measured that
corrosion potential of plate sample was more noble ( 47.62 mV) than in CT feed
water ( 71.43 mV). Also, the pitting resistance of plate samples in CT feed water
was higher (769.82 mV) than in bleed water (414.56 mV) due to higher concentra-
tion of chloride in it. The negative hysteresis in cyclic polarization depicted by stain-
less steel samples in auxiliary and jacket water corresponded to higher resistance to
pitting. The lower repassivation potential in bleed water by potentiodynamic polar-
ization was validated by the electrochemical scratch test (potentiostatic polarization)
by plate samples (Figure 2.11). The repassivation current density in CT feed and
2
2
bleed water was 3.13 μA/cm and 0.28 mA/cm , respectively.
3.2.6 Conclusion
The chemical analysis of metal plates was confirmed as 316L stainless steel and there
were no metallurgical inhomogeneities in it. The high level of chloride/chlorine in
open-circuit system (CT water) caused severe pitting and perforation in the heat-
exchanger plates. The low resistance to pitting by 316L plate samples in CT feed