Page 185 - Handbook of Plastics Technologies
P. 185
THERMOSETS
THERMOSETS 3.55
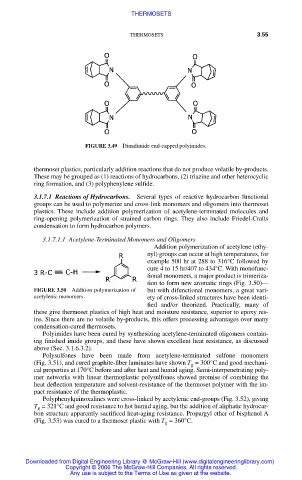
FIGURE 3.49 Dinadimide end-capped polyimides.
thermoset plastics, particularly addition reactions that do not produce volatile by-products.
These may be grouped as (1) reactions of hydrocarbons, (2) triazine and other heterocyclic
ring formation, and (3) polyphenylene sulfide.
3.1.7.1 Reactions of Hydrocarbons. Several types of reactive hydrocarbon functional
groups can be used to polymerize and cross-link monomers and oligomers into thermoset
plastics. These include addition polymerization of acetylene-terminated molecules and
ring-opening polymerization of strained carbon rings. They also include Friedel-Crafts
condensation to form hydrocarbon polymers.
3.1.7.1.1 Acetylene-Terminated Monomers and Oligomers
Addition polymerization of acetylene (ethy-
nyl) groups can occur at high temperatures, for
example 500 hr at 288 to 316°C followed by
cure 4 to 15 hr/407 to 434°C. With monofunc-
tional monomers, a major product is trimeriza-
tion to form new aromatic rings (Fig. 3.50)—
FIGURE 3.50 Addition polymerization of but with difunctional monomers, a great vari-
acetylenic monomers. ety of cross-linked structures have been identi-
fied and/or theorized. Practically, many of
these give thermoset plastics of high heat and moisture resistance, superior to epoxy res-
ins. Since there are no volatile by-products, this offers processing advantages over many
condensation-cured thermosets.
Polyimides have been cured by synthesizing acetylene-terminated oligomers contain-
ing finished imide groups, and these have shown excellent heat resistance, as discussed
above (Sec. 3.1.6.3.2).
Polysulfones have been made from acetylene-terminated sulfone monomers
(Fig. 3.51), and cured graphite-fiber laminates have shown T = 300°C and good mechani-
g
cal properties at 170°C before and after heat and humid aging. Semi-interpenetrating poly-
mer networks with linear thermoplastic polysulfones showed promise of combining the
heat deflection temperature and solvent-resistance of the thermoset polymer with the im-
pact resistance of the thermoplastic.
Polyphenylquinoxalines were cross-linked by acetylenic end-groups (Fig. 3.52), giving
T = 321°C and good resistance to hot humid aging, but the addition of aliphatic hydrocar-
g
bon structure apparently sacrificed heat-aging resistance. Propargyl ether of bisphenol A
(Fig. 3.53) was cured to a thermoset plastic with T = 360°C.
g
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.