Page 398 - Handbook of Properties of Textile and Technical Fibres
P. 398
The chemistry, manufacture, and tensile behavior of polyamide fibers 371
PA 6). The melting points (T m ) and glass transition temperatures (T g ) of polyamides
are increased with the concentration of amide groups (number of amide groups per
100 chain atoms) or are decreased in the number of CH 2 groups in their unit (mono-
mer), which corresponds to an increase of cohesive forces between adjacent molecules
(see Fig. 12.5). This tendency is not regular because the even or odd number of CH 2
groups between the NHeCO groups plays an important role (Puffr and Kubanek,
1991a). The odd AB polyamides have higher melting points than the even ones.
The density of polyamides increases with the concentration of amide groups. Most
of them have a similar zig-zag form. The melting points and densities depend on the
concentration of amide groups for polyamides prepared from dicarboxylic acids, and
diamines, and also from u-aminocarboxylic acids. It is suggested that odd or even
numbers of CH 2 groups, and their combinations, represent different molecular
alignments, i.e., different crystalline structure (Puffr and Kubanek, 1991a).
For fully extended chains, there can be all hydrogen bonds arranged for odd
polyamides in parallel and also in an antiparallel orientation. In the case of even
polyamides, the only possibility of complete hydrogen bonding in the sheets of
extended planar zig-zag chains is an antiparallel arrangement of neighboring chains
(Puffr and Kubanek, 1991a).
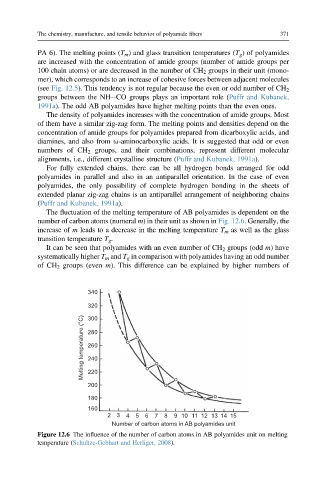
The fluctuation of the melting temperature of AB polyamides is dependent on the
number of carbon atoms (numeral m) in their unit as shown in Fig. 12.6. Generally, the
increase of m leads to a decrease in the melting temperature T m as well as the glass
transition temperature T g .
It can be seen that polyamides with an even number of CH 2 groups (odd m) have
systematically higher T m and T g in comparison with polyamides having an odd number
of CH 2 groups (even m). This difference can be explained by higher numbers of
340
320
Melting temperature (°C) 280
300
260
240
220
200
180
160
23 4 5 6 7 8 9 10 11 12 13 14 15
Number of carbon atoms in AB polyamides unit
Figure 12.6 The influence of the number of carbon atoms in AB polyamides unit on melting
temperature (Schultze-Gebhart and Herliger, 2008).