Page 399 - Handbook of Properties of Textile and Technical Fibres
P. 399
372 Handbook of Properties of Textile and Technical Fibres
C O CO
C O H N CO H N
H N HN
O C
O N H CO
N H C O HH
HN
PA 6
T = 225°C PA 7
m
CO T = 232°C
m
C O H N
HN CO
C O H N
HN
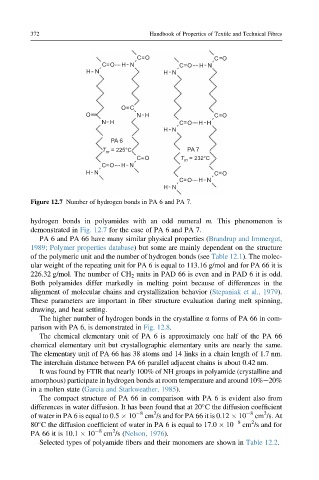
Figure 12.7 Number of hydrogen bonds in PA 6 and PA 7.
hydrogen bonds in polyamides with an odd numeral m. This phenomenon is
demonstrated in Fig. 12.7 for the case of PA 6 and PA 7.
PA 6 and PA 66 have many similar physical properties (Brandrup and Immergut,
1989; Polymer properties database) but some are mainly dependent on the structure
of the polymeric unit and the number of hydrogen bonds (see Table 12.1). The molec-
ular weight of the repeating unit for PA 6 is equal to 113.16 g/mol and for PA 66 it is
226.32 g/mol. The number of CH 2 units in PAD 66 is even and in PAD 6 it is odd.
Both polyamides differ markedly in melting point because of differences in the
alignment of molecular chains and crystallization behavior (Stepaniak et al., 1979).
These parameters are important in fiber structure evaluation during melt spinning,
drawing, and heat setting.
The higher number of hydrogen bonds in the crystalline a forms of PA 66 in com-
parison with PA 6, is demonstrated in Fig. 12.8.
The chemical elementary unit of PA 6 is approximately one half of the PA 66
chemical elementary unit but crystallographic elementary units are nearly the same.
The elementary unit of PA 66 has 38 atoms and 14 links in a chain length of 1.7 nm.
The interchain distance between PA 66 parallel adjacent chains is about 0.42 nm.
It was found by FTIR that nearly 100% of NH groups in polyamide (crystalline and
amorphous) participate in hydrogen bonds at room temperature and around 10%e20%
in a molten state (Garcia and Starkweather, 1985).
The compact structure of PA 66 in comparison with PA 6 is evident also from
differences in water diffusion. It has been found that at 20 C the diffusion coefficient
2
2
of water in PA 6 is equal to 0.5 10 8 cm /s and for PA 66 it is 0.12 10 8 cm /s. At
2
80 C the diffusion coefficient of water in PA 6 is equal to 17.0 10 8 cm /s and for
8 2
PA 66 it is 10.1 10 cm /s (Nelson, 1976).
Selected types of polyamide fibers and their monomers are shown in Table 12.2.