Page 497 - Handbook of Properties of Textile and Technical Fibres
P. 497
470 Handbook of Properties of Textile and Technical Fibres
It is known that M t is directly connected to the strength s B of PET fibers. The empir-
ical equation s B ¼ a þ b lnð M t þ cÞ, where a, b, and c are constants, has been pro-
posed in the work by Burgoyne and Merii (2007). The classical relation between
strength s B of PET fibers and M t has the form
(13.33)
s B ¼ A B=M t
where A is the limit strength for M t /N and B is coefficient of sensitivity.
A more complex model based on the fact that water absorption, and thus chain scis-
sion, are only possible in the amorphous phase, so that it is necessary to consider the
local concentrations of the water molecules in the amorphous phase, is presented in
Launay et al. (1994). For this model the rate of chain scission is described by the
equation
dN b
¼ K s ðN cg N b ÞðW 0 þ cN b Þ (13.34)
dt
where constants W 0 ¼ 0.75 mol/kg and c ¼ 0.179 mol/kg were obtained from the
experimental dependence between W and the number of chain scissions N b , per unit
mass of the amorphous phase (Launay et al., 1994).
For practical use of technical PET fibers it is important to estimate strength loss due
to hydrolysis. Generally, polyester fibers will hydrolyze within a wide range of pH.
This reduces the polymer chain length and the strength of the fiber. Hydrolysis in
þ
neutral and acid conditions is due to the attack of H ions. In acidic conditions accel-
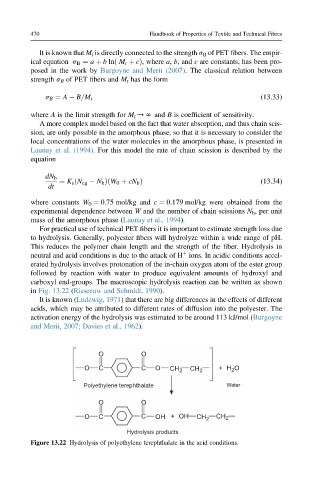
erated hydrolysis involves protonation of the in-chain oxygen atom of the ester group
followed by reaction with water to produce equivalent amounts of hydroxyl and
carboxyl end-groups. The macroscopic hydrolysis reaction can be written as shown
in Fig. 13.22 (Risseeuw and Schmidt, 1990).
It is known (Ludewig, 1971) that there are big differences in the effects of different
acids, which may be attributed to different rates of diffusion into the polyester. The
activation energy of the hydrolysis was estimated to be around 113 kJ/mol (Burgoyne
and Merii, 2007; Davies et al., 1962).
O O
O C C O CH 2 CH 2 + H O
2
Polyethylene terephthalate Water
O O
O C C OH + OH CH 2 CH 2
Hydrolysis products
Figure 13.22 Hydrolysis of polyethylene terephthalate in the acid conditions.