Page 498 - Handbook of Properties of Textile and Technical Fibres
P. 498
Tensile failure of polyester fibers 471
O O
H O C C O CH 2 CH 2 OH + 2n(NaOH)
n
Polyethylene terephthalate Sodium hydroxide
O O
n Na O C C O Na + n(HO CH 2 CH OH)
2
Disodium terephthalate Ethylene glycol
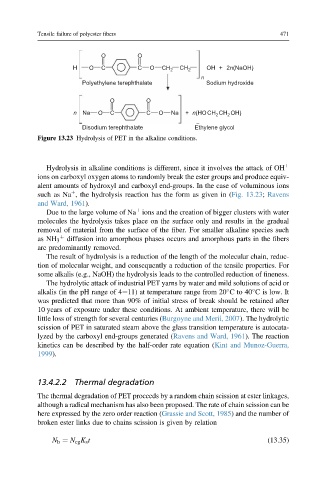
Figure 13.23 Hydrolysis of PET in the alkaline conditions.
Hydrolysis in alkaline conditions is different, since it involves the attack of OH þ
ions on carboxyl oxygen atoms to randomly break the ester groups and produce equiv-
alent amounts of hydroxyl and carboxyl end-groups. In the case of voluminous ions
þ
such as Na , the hydrolysis reaction has the form as given in (Fig. 13.23; Ravens
and Ward, 1961).
þ
Due to the large volume of Na ions and the creation of bigger clusters with water
molecules the hydrolysis takes place on the surface only and results in the gradual
removal of material from the surface of the fiber. For smaller alkaline species such
þ diffusion into amorphous phases occurs and amorphous parts in the fibers
as NH 3
are predominantly removed.
The result of hydrolysis is a reduction of the length of the molecular chain, reduc-
tion of molecular weight, and consequently a reduction of the tensile properties. For
some alkalis (e.g., NaOH) the hydrolysis leads to the controlled reduction of fineness.
The hydrolytic attack of industrial PET yarns by water and mild solutions of acid or
alkalis (in the pH range of 4e11) at temperature range from 20 Cto40 C is low. It
was predicted that more than 90% of initial stress of break should be retained after
10 years of exposure under these conditions. At ambient temperature, there will be
little loss of strength for several centuries (Burgoyne and Merii, 2007). The hydrolytic
scission of PET in saturated steam above the glass transition temperature is autocata-
lyzed by the carboxyl end-groups generated (Ravens and Ward, 1961). The reaction
kinetics can be described by the half-order rate equation (Kint and Munoz-Guerra,
1999).
13.4.2.2 Thermal degradation
The thermal degradation of PET proceeds by a random chain scission at ester linkages,
although a radical mechanism has also been proposed. The rate of chain scission can be
here expressed by the zero order reaction (Grassie and Scott, 1985) and the number of
broken ester links due to chains scission is given by relation
N b ¼ N cg K s t (13.35)