Page 519 - Handbook of Properties of Textile and Technical Fibres
P. 519
492 Handbook of Properties of Textile and Technical Fibres
emitting near 360 nm (Ouchi et al., 1976). PEN possesses electrical insulation proper-
ties that are on average 25% better than PET.
PEN materials are thermally and mechanically much better in comparison with PET
but an insurmountable problem for a long time was the inaccessibility of the 2,6-NDA
(Ozerenko et al., 2007). Within the last few years, Amoco and Mitsubishi have been
able to supply the dimethyl ester of NDA on a commercial scale at competitive prices.
The NDA is made by the cobalt manganese catalyzed air oxidation of 2,6-dimethyl-
naphthalene synthesized from monocyclic benzene derivatives. PEN polymer chemis-
try and its major applications areas were published by Sakellarides (2004). PEN fibers
were commercialized in 2002 by Honeywell Performance Fibers under the name
Pentex.
The design of reliable catalysts for the synthesis of NDA on the basis of coke
industry naphthalene and its methyl-substituted derivatives is described by Ozerenko
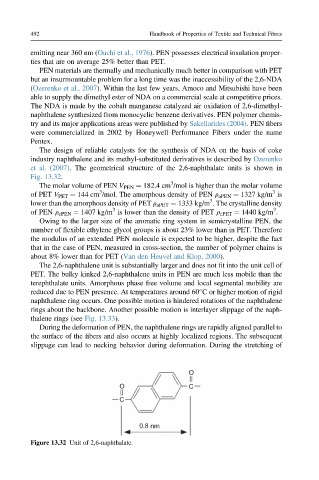
et al. (2007). The geometrical structure of the 2,6-naphthalate units is shown in
Fig. 13.32.
3
The molar volume of PEN V PEN ¼ 182.4 cm /mol is higher than the molar volume
3
3
of PET V PET ¼ 144 cm /mol. The amorphous density of PEN r aPEN ¼ 1327 kg/m is
3
lower than the amorphous density of PET r aPET ¼ 1333 kg/m . The crystalline density
3
3
of PEN r cPEN ¼ 1407 kg/m is lower than the density of PET r cPET ¼ 1440 kg/m .
Owing to the larger size of the aromatic ring system in semicrystalline PEN, the
number of flexible ethylene glycol groups is about 23% lower than in PET. Therefore
the modulus of an extended PEN molecule is expected to be higher, despite the fact
that in the case of PEN, measured in cross-section, the number of polymer chains is
about 8% lower than for PET (Van den Heuvel and Klop, 2000).
The 2,6-naphthalene unit is substantially larger and does not fit into the unit cell of
PET. The bulky kinked 2,6-naphthalene units in PEN are much less mobile than the
terephthalate units. Amorphous phase free volume and local segmental mobility are
reduced due to PEN presence. At temperatures around 60 C or higher motion of rigid
naphthalene ring occurs. One possible motion is hindered rotations of the naphthalene
rings about the backbone. Another possible motion is interlayer slippage of the naph-
thalene rings (see Fig. 13.33).
During the deformation of PEN, the naphthalene rings are rapidly aligned parallel to
the surface of the fibers and also occurs at highly localized regions. The subsequent
slippage can lead to necking behavior during deformation. During the stretching of
O
O C
C
0.8 nm
Figure 13.32 Unit of 2,6-naphthalate.