Page 207 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 207
184 High Temperature Solid Oxide Fuel Cells: Fundamentals. Design and Applications
Lao.6Sro.4Feo.8Coo.203 strongly increased with time for those steels containing
A1 and Si in the range 1-2 wt%. Lowest contact resistances were obtained with
X3 CrTi 17 and X2 CrTiNb 18 steels, remaining below 10 mS2 cm2 after 4000 h of
exposure in air. In corrosion experiments, both of these steels formed scales
composed of chromia and Fe-Mn-Cr spineIs together with an internal oxidation
of the stabilising elements Ti and Nb [ 541.
Further progress has been made in developing ferritic steels that form thin
spinel-type corrosion scales with significant electrical conductivity and have
well-adherent corrosion scales which reduce the release of volatile Cr species
[5 5,561. By adding various alloying elements in the range 0.1-2.5 wt% to alloys
with 17-25 wt% chromium, it was learned that:
0 Ni does not support a stable and protective scale formation
0 Ti leads to higher oxidation rate due to enhanced growth rate of the
chromia scale and formation of internal Ti oxides
0 Y, La, Ce, and Zr reduce the oxide growth rate independent of Cr content:
especially La promotes very thin oxide scales
0 Mn increases the oxide scale growth rate even if a lanthanide element is
present, and preferentially forms a Cr-Mn spinel with low electrical
resistance on top of achromia scale
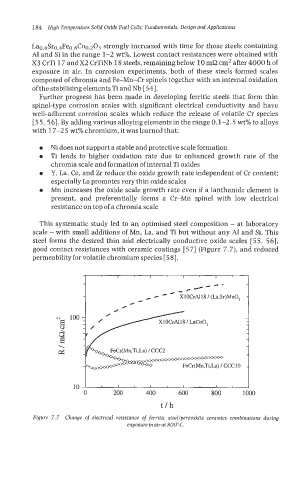
This systematic study led to an optimised steel composition - at laboratory
scale - with small additions of Mn, La, and Ti but without any A1 and Si. This
steel forms the desired thin and electrically conductive oxide scales [55, 561,
good contact resistances with ceramic coatings [5 71 (Figure 7.7), and reduced
permeability for volatile chromium species [58].
1 I I I I
-E
\ ii
0 200 400 600 800 1000
t/h
Figure 7.7 Change of electrical resistance of ferritic steellperovskite ceramics combinations during
exposureinairat 800°C.