Page 124 - Hydrocarbon Exploration and Production Second Edition
P. 124
Reservoir Description 111
Those with one carbon–carbon double bond are called mono-olefins or alkenes,for
example ethylene CH 2 QCH 2 .
The double bond is not stronger than the single bond; on the contrary, it is more
vulnerable, making unsaturated compounds more chemically reactive than the
saturates.
In the longer carbon chains, two carbon–carbon double bonds may exist. Such
molecules are called diolefins (or dienes), such as butadiene CH 2 QCH–CHQCH 2 .
6.2.1.3. Acetylenes
Acetylenes are another series of unsaturated hydrocarbons which include com-
pounds containing a carbon–carbon triple bond, for example acetylene itself:
CHRCH
Olefins are uncommon in crude oils due to the high chemical activity of these
compounds which causes them to become saturated with hydrogen. Similarly,
acetylene is virtually absent from crude oil, which tends to contain a large proportion
of the saturated hydrocarbons, such as the alkanes.
Whilst the long-chain hydrocarbons (above 18 carbon atoms) may exist in
solution at reservoir temperature and pressure, they can solidify at the lower
temperatures and pressures experienced in surface facilities, or even in the tubing.
The fraction of the longer chain hydrocarbons in the crude oil is therefore of
particular interest to process engineers, who will typically require a detailed
laboratory analysis of the crude oil composition, extending to the measurement of
the fraction of molecules as long as C 30 .
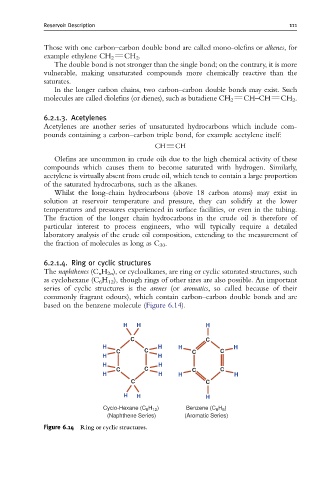
6.2.1.4. Ring or cyclic structures
The naphthenes (C n H 2n ), or cycloalkanes, are ring or cyclic saturated structures, such
as cyclohexane (C 6 H 12 ), though rings of other sizes are also possible. An important
series of cyclic structures is the arenes (or aromatics, so called because of their
commonly fragrant odours), which contain carbon–carbon double bonds and are
based on the benzene molecule (Figure 6.14).
H H H
C C
H H H H
C C C C
H H
H H
C C C C
H C H H C H
H H
H
Cyclo-Hexane (C 6 H 12 ) Benzene (C 6 H 6 )
(Naphthene Series) (Aromatic Series)
Figure 6.14 Ring or cyclic structures.