Page 125 - Hydrocarbon Exploration and Production Second Edition
P. 125
112 Reservoir Fluids
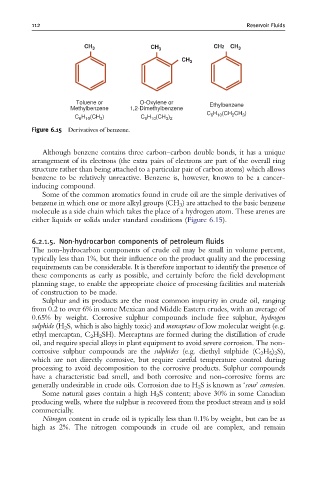
CH 3 CH 3 CH2 CH 3
CH 3
Toluene or O-Oxylene or Ethylbenzene
Methylbenzene 1,2-Dimethylbenzene
C H (CH CH )
H (CH )
C H (CH ) C 6 10 3 2 6 10 2 3
3
6 10
Figure 6.15 Derivatives of benzene.
Although benzene contains three carbon–carbon double bonds, it has a unique
arrangement of its electrons (the extra pairs of electrons are part of the overall ring
structure rather than being attached to a particular pair of carbon atoms) which allows
benzene to be relatively unreactive. Benzene is, however, known to be a cancer-
inducing compound.
Some of the common aromatics found in crude oil are the simple derivatives of
benzene in which one or more alkyl groups (CH 3 ) are attached to the basic benzene
molecule as a side chain which takes the place of a hydrogen atom. These arenes are
either liquids or solids under standard conditions (Figure 6.15).
6.2.1.5. Non-hydrocarbon components of petroleum fluids
The non-hydrocarbon components of crude oil may be small in volume percent,
typically less than 1%, but their influence on the product quality and the processing
requirements can be considerable. It is therefore important to identify the presence of
these components as early as possible, and certainly before the field development
planning stage, to enable the appropriate choice of processing facilities and materials
of construction to be made.
Sulphur and its products are the most common impurity in crude oil, ranging
from 0.2 to over 6% in some Mexican and Middle Eastern crudes, with an average of
0.65% by weight. Corrosive sulphur compounds include free sulphur, hydrogen
sulphide (H 2 S, which is also highly toxic) and mercaptans of low molecular weight (e.g.
ethyl mercaptan, C 2 H 2 SH). Mercaptans are formed during the distillation of crude
oil, and require special alloys in plant equipment to avoid severe corrosion. The non-
corrosive sulphur compounds are the sulphides (e.g. diethyl sulphide (C 2 H 5 ) 2 S),
which are not directly corrosive, but require careful temperature control during
processing to avoid decomposition to the corrosive products. Sulphur compounds
have a characteristic bad smell, and both corrosive and non-corrosive forms are
generally undesirable in crude oils. Corrosion due to H 2 S is known as ‘sour’ corrosion.
Some natural gases contain a high H 2 S content; above 30% in some Canadian
producing wells, where the sulphur is recovered from the product stream and is sold
commercially.
Nitrogen content in crude oil is typically less than 0.1% by weight, but can be as
high as 2%. The nitrogen compounds in crude oil are complex, and remain