Page 128 - Hydrocarbon Exploration and Production Second Edition
P. 128
Reservoir Description 115
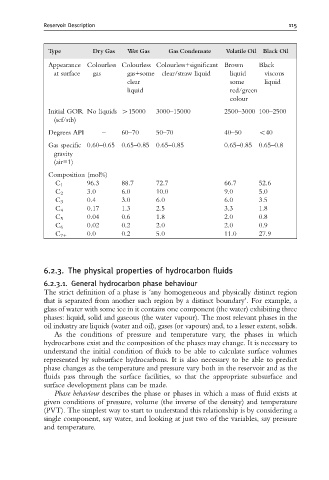
Type Dry Gas Wet Gas Gas Condensate Volatile Oil Black Oil
Appearance Colourless Colourless Colourless+significant Brown Black
at surface gas gas+some clear/straw liquid liquid viscous
clear some liquid
liquid red/green
colour
Initial GOR No liquids W15000 3000–15000 2500–3000 100–2500
(scf/stb)
Degrees API – 60–70 50–70 40–50 o40
Gas specific 0.60–0.65 0.65–0.85 0.65–0.85 0.65–0.85 0.65–0.8
gravity
(air=1)
Composition (mol%)
96.3 88.7 72.7 66.7 52.6
C 1
3.0 6.0 10.0 9.0 5.0
C 2
0.4 3.0 6.0 6.0 3.5
C 3
C 4 0.17 1.3 2.5 3.3 1.8
C 5 0.04 0.6 1.8 2.0 0.8
0.02 0.2 2.0 2.0 0.9
C 6
0.0 0.2 5.0 11.0 27.9
C 7+
6.2.3. The physical properties of hydrocarbon fluids
6.2.3.1. General hydrocarbon phase behaviour
The strict definition of a phase is ‘any homogeneous and physically distinct region
that is separated from another such region by a distinct boundary’. For example, a
glass of water with some ice in it contains one component (the water) exhibiting three
phases: liquid, solid and gaseous (the water vapour). The most relevant phases in the
oil industry are liquids (water and oil), gases (or vapours) and, to a lesser extent, solids.
As the conditions of pressure and temperature vary, the phases in which
hydrocarbons exist and the composition of the phases may change. It is necessary to
understand the initial condition of fluids to be able to calculate surface volumes
represented by subsurface hydrocarbons. It is also necessary to be able to predict
phase changes as the temperature and pressure vary both in the reservoir and as the
fluids pass through the surface facilities, so that the appropriate subsurface and
surface development plans can be made.
Phase behaviour describes the phase or phases in which a mass of fluid exists at
given conditions of pressure, volume (the inverse of the density) and temperature
(PVT). The simplest way to start to understand this relationship is by considering a
single component, say water, and looking at just two of the variables, say pressure
and temperature.