Page 110 - Hydrogeology Principles and Practice
P. 110
HYDC03 12/5/05 5:36 PM Page 93
Chemical hydrogeology 93
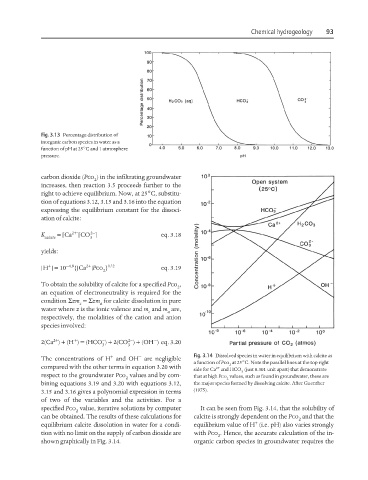
Fig. 3.13 Percentage distribution of
inorganic carbon species in water as a
function of pH at 25°C and 1 atmosphere
pressure.
carbon dioxide (Pco ) in the infiltrating groundwater
2
increases, then reaction 3.5 proceeds further to the
right to achieve equilibrium. Now, at 25°C, substitu-
tion of equations 3.12, 3.15 and 3.16 into the equation
expressing the equilibrium constant for the dissoci-
ation of calcite:
2+ 2−
K = [Ca ][CO ] eq. 3.18
calcite 3
yields:
+ −4.9 2+ 1/2
[H ] = 10 {[Ca ]Pco } eq. 3.19
2
To obtain the solubility of calcite for a specified Pco ,
2
an equation of electroneutrality is required for the
condition Σzm =Σzm for calcite dissolution in pure
c a
water where z is the ionic valence and m and m are,
c a
respectively, the molalities of the cation and anion
species involved:
2+ + − 2− −
2(Ca ) + (H ) = (HCO ) + 2(CO ) + (OH ) eq. 3.20
3 3
+ − Fig. 3.14 Dissolved species in water in equilibrium with calcite as
The concentrations of H and OH are negligible
a function of Pco at 25°C. Note the parallel lines at the top right
compared with the other terms in equation 3.20 with 2+ 2 −
side for Ca and HCO (just 0.301 unit apart) that demonstrate
3
respect to the groundwater Pco values and by com- that at high Pco values, such as found in groundwater, these are
2 2
bining equations 3.19 and 3.20 with equations 3.12, the major species formed by dissolving calcite. After Guenther
3.15 and 3.16 gives a polynomial expression in terms (1975).
of two of the variables and the activities. For a
specified Pco value, iterative solutions by computer It can be seen from Fig. 3.14, that the solubility of
2
can be obtained. The results of these calculations for calcite is strongly dependent on the Pco and that the
2
+
equilibrium calcite dissolution in water for a condi- equilibrium value of H (i.e. pH) also varies strongly
tion with no limit on the supply of carbon dioxide are with Pco . Hence, the accurate calculation of the in-
2
shown graphically in Fig. 3.14. organic carbon species in groundwater requires the