Page 115 - Hydrogeology Principles and Practice
P. 115
HYDC03 12/5/05 5:36 PM Page 98
98 Chapter Three
(a) Open-system However, if both minerals occur in a hydrogeological
system they may both dissolve simultaneously or
sequentially leading to different equilibrium rela-
Recharge tions compared to those shown in Fig. 3.16. In this
situation, a comparison of equilibrium constants for
Soil zone.
CO 2 from oxidation of organic matter and plant root respiration calcite and dolomite for the particular groundwater
temperature is necessary to define which mineral is
⇒ constant PCO 2
dissolving incongruently.
For example, considering the thermodynamic
data shown in Table 3.6, at about 20°C, K =
calcite
Soil water equilibrates with CO 2 and dissolves CaCO 3 in soil material K 1/2 . Under these conditions, since the solubility
dolomite
2−
2+
product (Box 3.6) of calcite is equal to [Ca ][CO ]
3
2+ 2+ 2−
Calcite saturation achieved infiltrating groundwater and for dolomite is equal to [Ca ][Mg ][CO ]
3
2−
[CO ], if groundwater saturated with dolomite
Aquifter 3
flows into a zone that contains calcite, no calcite dis-
(b) Closed-system
solution will occur because the water is already satur-
ated with respect to calcite. At temperatures lower
than 20°C, K < K 1/2 and if groundwater dis-
Recharge calcite dolomite
solves dolomite to equilibrium the water becomes
Soil zone:
Soil water equilibrates with CO 2 carbonate-free soil material supersaturated with respect to calcite which can then
precipitate. In a system where the rate of dolomite
dissolution is equal to the rate of calcite precipitation,
Infiltrating groundwater containing CO 2
this is the condition of incongruent dissolution of
Aquifer carbonate encountered in dolomite. At temperatures higher than 20°C, K calcite >
the saturated zone 1/2
K and if dolomite saturation is achieved with
Calcite dissolution with no dolomite
the groundwater then entering a region containing
replenishment of CO 2
Calcite saturation achieved calcite, calcite dissolution will occur leading to an
2+ 2−
increase in Ca and CO concentrations. The water
3
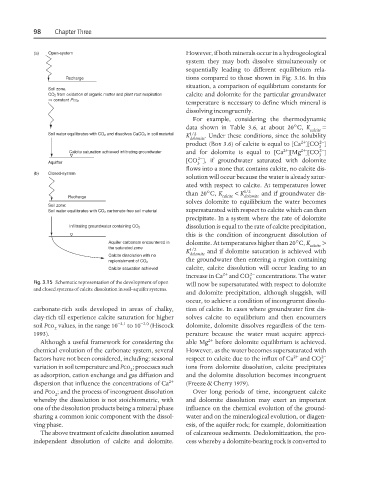
Fig. 3.15 Schematic representation of the development of open
will now be supersaturated with respect to dolomite
and closed systems of calcite dissolution in soil–aquifer systems.
and dolomite precipitation, although sluggish, will
occur, to achieve a condition of incongruent dissolu-
carbonate-rich soils developed in areas of chalky, tion of calcite. In cases where groundwater first dis-
clay-rich till experience calcite saturation for higher solves calcite to equilibrium and then encounters
soil Pco values, in the range 10 −2.1 to 10 −2.0 (Hiscock dolomite, dolomite dissolves regardless of the tem-
2
1993). perature because the water must acquire appreci-
2+
Although a useful framework for considering the able Mg before dolomite equilibrium is achieved.
chemical evolution of the carbonate system, several However, as the water becomes supersaturated with
2+ 2−
factors have not been considered, including: seasonal respect to calcite due to the influx of Ca and CO
3
variation in soil temperature and Pco ; processes such ions from dolomite dissolution, calcite precipitates
2
as adsorption, cation exchange and gas diffusion and and the dolomite dissolution becomes incongruent
2+
dispersion that influence the concentrations of Ca (Freeze & Cherry 1979).
and Pco ; and the process of incongruent dissolution Over long periods of time, incongruent calcite
2
whereby the dissolution is not stoichiometric, with and dolomite dissolution may exert an important
one of the dissolution products being a mineral phase influence on the chemical evolution of the ground-
sharing a common ionic component with the dissol- water and on the mineralogical evolution, or diagen-
ving phase. esis, of the aquifer rock; for example, dolomitization
The above treatment of calcite dissolution assumed of calcareous sediments. Dedolomitization, the pro-
independent dissolution of calcite and dolomite. cess whereby a dolomite-bearing rock is converted to