Page 119 - Hydrogeology Principles and Practice
P. 119
HYDC03 12/5/05 5:36 PM Page 102
102 Chapter Three
Table 3.6 Thermodynamic equilibrium constants for calcite, dolomite and major aqueous carbonate species in pure water for a
temperature range of 0–30°C and 1 atmosphere total pressure. Note that pK =−log K. Thermodynamic data from Langmuir (1971) and
10
Plummer and Busenberg (1982).
Temperature (°C) pK pK pK − pK pK K /(K ) 1/2
CO 2 H 2 CO 3 HCO 3 calcite dolomite calcite dolomite
0 1.11 6.58 10.63 8.38 16.18 0.51
5 1.19 6.52 10.56 8.39 16.39 0.63
10 1.27 6.47 10.49 8.41 16.57 0.75
15 1.34 6.42 10.43 8.43 16.74 0.87
20 1.41 6.38 10.38 8.45 16.88 0.97
25 1.47 6.35 10.33 8.48 17.00 1.05
30 1.52 6.33 10.29 8.51 17.11 1.11
centration of cations in solution and the energy of
adsorption of the cations at the exchange surface. The
monovalent ions have a smaller energy of adsorption
and are therefore more likely to remain in solution.
As a result of a larger energy of adsorption, divalent
ions are more abundant as exchangeable cations.
2+
Ca is typically more abundant as an exchangeable
2+ + +
cation than is Mg , K or Na . The energy absorp-
2+ 2+ + +
tion sequence is: Ca > Mg > K > Na and this
provides a general ordering of cation exchangeability
for common ions in groundwater.
Values of CEC are found experimentally with lab-
−1
oratory results reported in terms of meq (100 g) .
CEC is commonly determined by extraction of the
cations from soils or aquifer materials with a solution
+
containing a known cation, normally NH . Ammon-
4
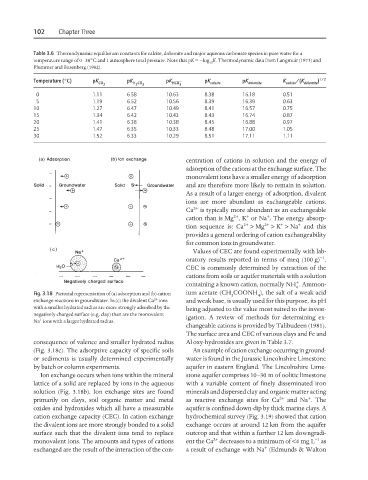
Fig. 3.18 Pictorial representation of (a) adsorption and (b) cation ium acetate (CH COONH ), the salt of a weak acid
4
3
2+
exchange reactions in groundwater. In (c) the divalent Ca ions and weak base, is usually used for this purpose, its pH
with a smaller hydrated radius are more strongly adsorbed by the being adjusted to the value most suited to the invest-
negatively charged surface (e.g. clay) than are the monovalent
+ igation. A review of methods for determining ex-
Na ions with a larger hydrated radius.
changeable cations is provided by Talibudeen (1981).
The surface area and CEC of various clays and Fe and
consequence of valence and smaller hydrated radius Al oxy-hydroxides are given in Table 3.7.
(Fig. 3.18c). The adsorptive capacity of specific soils An example of cation exchange occurring in ground-
or sediments is usually determined experimentally water is found in the Jurassic Lincolnshire Limestone
by batch or column experiments. aquifer in eastern England. The Lincolnshire Lime-
Ion exchange occurs when ions within the mineral stone aquifer comprises 10–30 m of oolitic limestone
lattice of a solid are replaced by ions in the aqueous with a variable content of finely disseminated iron
solution (Fig. 3.18b). Ion exchange sites are found minerals and dispersed clay and organic matter acting
2+ +
primarily on clays, soil organic matter and metal as reactive exchange sites for Ca and Na . The
oxides and hydroxides which all have a measurable aquifer is confined down-dip by thick marine clays. A
cation exchange capacity (CEC). In cation exchange hydrochemical survey (Fig. 3.19) showed that cation
the divalent ions are more strongly bonded to a solid exchange occurs at around 12 km from the aquifer
surface such that the divalent ions tend to replace outcrop and that within a further 12 km downgradi-
2+ −1
monovalent ions. The amounts and types of cations ent the Ca decreases to a minimum of <4mgL as
+
exchanged are the result of the interaction of the con- a result of exchange with Na (Edmunds & Walton