Page 123 - Hydrogeology Principles and Practice
P. 123
HYDC03 12/5/05 5:37 PM Page 106
106 Chapter Three
BO X
Continued
3.7
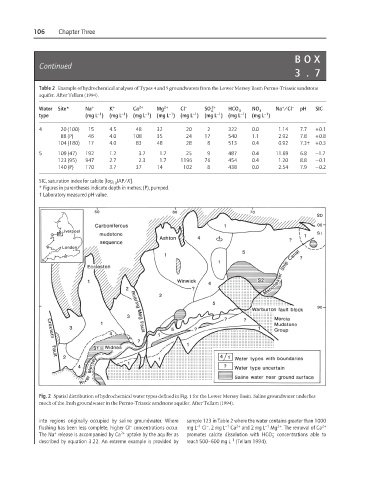
Table 2 Example of hydrochemical analyses of Types 4 and 5 groundwaters from the Lower Mersey Basin Permo-Triassic sandstone
aquifer. After Tellam (1994).
+
Water Site* Na + K + Ca 2+ Mg 2+ Cl − SO 2+ HCO − NO − Na /Cl − pH SIC
4 3 3
−1
−1
−1
−1
−1
−1
−1
−1
type (mg L ) (mg L ) (mg L ) (mg L ) (mg L ) (mg L ) (mg L ) (mg L )
4 20 (100) 15 4.5 48 32 20 2 322 0.0 1.14 7.7 +0.1
88 (P) 46 4.0 108 35 24 17 540 1.1 2.92 7.8 +0.8
104 (180) 17 4.0 83 48 28 8 513 0.4 0.92 7.3† +0.3
5 109 (47) 192 1.2 3.7 1.7 25 9 487 0.4 11.69 6.8 −1.7
123 (95) 947 2.7 2.3 1.7 1196 76 454 0.4 1.20 8.8 −0.1
140 (P) 170 3.7 37 14 102 8 438 0.0 2.54 7.9 −0.2
SIC, saturation index for calcite [log IAP/K].
10
* Figures in parentheses indicate depth in metres; (P), pumped.
† Laboratory measured pH value.
Fig. 2 Spatial distribution of hydrochemical water types defined in Fig. 1 for the Lower Mersey Basin. Saline groundwater underlies
much of the fresh groundwater in the Permo-Triassic sandstone aquifer. After Tellam (1994).
into regions originally occupied by saline groundwater. Where sample 123 in Table 2 where the water contains greater than 1000
2+
−
2+
−
−1
−1
−1
flushing has been less complete, higher Cl concentrations occur. mg L Cl , 2mgL Ca and 2 mg L Mg . The removal of Ca 2+
−
+
The Na release is accompanied by Ca 2+ uptake by the aquifer as promotes calcite dissolution with HCO concentrations able to
3
−1
described by equation 3.22. An extreme example is provided by reach 500–600 mg L (Tellam 1994).