Page 121 - Hydrogeology Principles and Practice
P. 121
HYDC03 12/5/05 5:37 PM Page 104
104 Chapter Three
c = c − c eq. 3.24 c = f · c + (1 − f )c eq. 3.25
i,react i,sample i,mix Na,mix saline Na,saline saline Na,fresh
−
As an example calculation, the data shown in Now, using the mixed and saline Cl concentration
Table 3.8 are for samples 0, 14 and 19 of Fig. 3.19 values (samples 14 and 19) to indicate the fraction of
representing fresh, mixed and saline groundwaters the saline water ( f = 114/1100), and assuming a
saline
−
present in the Lincolnshire Limestone aquifer. To cal- freshwater end-member Cl concentration value equal
+
culate how much Na has been added to the mixed to zero, then substituting the values from Table 3.8
groundwater sample by cation exchange, equation into equation 3.25:
3.23 can be re-written as:
Cation exchange in the Lower Mersey Basin Permo-Triassic sandstone BO X
aquifer, England 3.7
The Lower Mersey Basin Permo-Triassic sandstone aquifer of north-
west England demonstrates the effect of very long-term natural
flushing of a saline aquifer. The aquifer comprises two main units:
the Permian Collyhurst Sandstone Formation and the Triassic
Sherwood Sandstone Formation that dip southwards at about 5°
and are up to 500 m thick. To the south, the aquifer unit is overlain
by the Triassic Mercia Mudstone Group, a formation which contains
evaporites. Underlying the aquifer is a Permian sequence, the upper
formations of which are of low permeability that rest uncon-
formably on Carboniferous mudstones. The sequence is extensively
faulted with throws frequently in excess of 100 m. Overlying the
older formations are highly heterogeneous, vertically variable
Quaternary deposits dominated by glacial till. Pumping of the
aquifer system has caused a decline in water levels such that much
of the sandstone aquifer is no longer confined by the till.
Typical compositions of the sandstones are quartz 60–70%,
feldspar 3–6%, lithic clasts 8%, calcite 0–10% and clays (including
smectite) <15%. Haematite imparts a red colour to most of the
sequence. The sandstones contain thin mudstone beds often less
than 10 cm in thickness. Cation exchange capacities are of the
−1
order of 1 meq (100 g) . Underlying the fresh groundwaters pre-
−
sent in the area are saline groundwaters attaining a Cl concentra-
−1
tion of up to 100 g L , which appear to be derived from dissolution
of evaporites in the overlying Mercia Mudstone Group (Tellam
1995). This saline water is present within 50 m of ground level
immediately up-flow of the Warburton Fault Block and along the
Mersey Valley but the freshwater–saline water interface is found
deeper both to the north and south (Tellam et al. 1986).
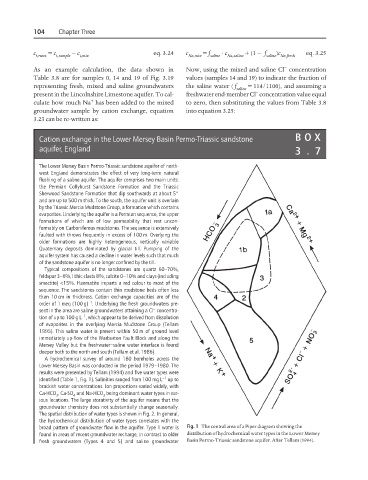
A hydrochemical survey of around 180 boreholes across the
Lower Mersey Basin was conducted in the period 1979–1980. The
results were presented by Tellam (1994) and five water types were
−1
identified (Table 1, Fig. 1). Salinities ranged from 100 mg L up to
brackish water concentrations. Ion proportions varied widely, with
Ca-HCO , Ca-SO and Na-HCO being dominant water types in var-
3
3
4
ious locations. The large storativity of the aquifer means that the
groundwater chemistry does not substantially change seasonally.
The spatial distribution of water types is shown in Fig. 2. In general,
the hydrochemical distribution of water types correlates with the
broad pattern of groundwater flow in the aquifer. Type 1 water is Fig. 1 The central area of a Piper diagram showing the
found in areas of recent groundwater recharge, in contrast to older distribution of hydrochemical water types in the Lower Mersey
fresh groundwaters (Types 4 and 5) and saline groundwater Basin Permo-Triassic sandstone aquifer. After Tellam (1994).