Page 116 - Hydrogeology Principles and Practice
P. 116
HYDC03 12/5/05 5:36 PM Page 99
Chemical hydrogeology 99
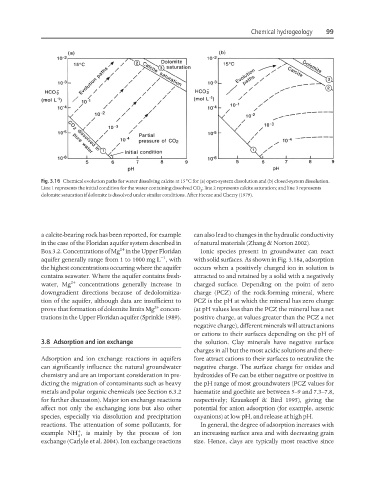
Fig. 3.16 Chemical evolution paths for water dissolving calcite at 15°C for (a) open-system dissolution and (b) closed-system dissolution.
Line 1 represents the initial condition for the water containing dissolved CO ; line 2 represents calcite saturation; and line 3 represents
2
dolomite saturation if dolomite is dissolved under similar conditions. After Freeze and Cherry (1979).
a calcite-bearing rock has been reported, for example can also lead to changes in the hydraulic conductivity
in the case of the Floridan aquifer system described in of natural materials (Zhang & Norton 2002).
2+
Box 3.2. Concentrations of Mg in the Upper Floridan Ionic species present in groundwater can react
−1
aquifer generally range from 1 to 1000 mg L , with with solid surfaces. As shown in Fig. 3.18a, adsorption
the highest concentrations occurring where the aquifer occurs when a positively charged ion in solution is
contains seawater. Where the aquifer contains fresh- attracted to and retained by a solid with a negatively
2+
water, Mg concentrations generally increase in charged surface. Depending on the point of zero
downgradient directions because of dedolomitiza- charge (PCZ) of the rock-forming mineral, where
tion of the aquifer, although data are insufficient to PCZ is the pH at which the mineral has zero charge
2+
prove that formation of dolomite limits Mg concen- (at pH values less than the PCZ the mineral has a net
trations in the Upper Floridan aquifer (Sprinkle 1989). positive charge, at values greater than the PCZ a net
negative charge), different minerals will attract anions
or cations to their surfaces depending on the pH of
3.8 Adsorption and ion exchange the solution. Clay minerals have negative surface
charges in all but the most acidic solutions and there-
Adsorption and ion exchange reactions in aquifers fore attract cations to their surfaces to neutralize the
can significantly influence the natural groundwater negative charge. The surface charge for oxides and
chemistry and are an important consideration in pre- hydroxides of Fe can be either negative or positive in
dicting the migration of contaminants such as heavy the pH range of most groundwaters (PCZ values for
metals and polar organic chemicals (see Section 6.3.2 haematite and goethite are between 5–9 and 7.3–7.8,
for further discussion). Major ion exchange reactions respectively; Krauskopf & Bird 1995), giving the
affect not only the exchanging ions but also other potential for anion adsorption (for example, arsenic
species, especially via dissolution and precipitation oxyanions) at low pH, and release at high pH.
reactions. The attenuation of some pollutants, for In general, the degree of adsorption increases with
+
example NH , is mainly by the process of ion an increasing surface area and with decreasing grain
4
exchange (Carlyle et al. 2004). Ion exchange reactions size. Hence, clays are typically most reactive since