Page 105 - Hydrogeology Principles and Practice
P. 105
HYDC03 12/5/05 5:36 PM Page 88
88 Chapter Three
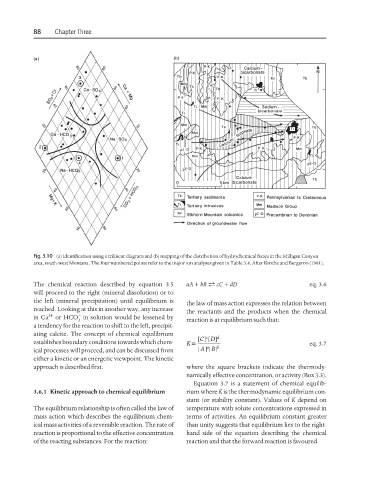
Fig. 3.10 (a) Identification using a trilinear diagram and (b) mapping of the distribution of hydrochemical facies in the Milligan Canyon
area, south-west Montana. The four numbered points refer to the major ion analyses given in Table 3.4. After Krothe and Bergeron (1981).
The chemical reaction described by equation 3.5 aA + bB j cC + dD eq. 3.6
will proceed to the right (mineral dissolution) or to
the left (mineral precipitation) until equilibrium is the law of mass action expresses the relation between
reached. Looking at this in another way, any increase the reactants and the products when the chemical
2+ −
in Ca or HCO in solution would be lessened by
3 reaction is at equilibrium such that:
a tendency for the reaction to shift to the left, precipit-
ating calcite. The concept of chemical equilibrium
c
C [] [ D] d
establishes boundary conditions towards which chem- K = eq. 3.7
a
ical processes will proceed, and can be discussed from A [] [ B] b
either a kinetic or an energetic viewpoint. The kinetic
approach is described first. where the square brackets indicate the thermody-
namically effective concentration, or activity (Box 3.3).
Equation 3.7 is a statement of chemical equilib-
3.6.1 Kinetic approach to chemical equilibrium rium where K is the thermodynamic equilibrium con-
stant (or stability constant). Values of K depend on
The equilibrium relationship is often called the law of temperature with solute concentrations expressed in
mass action which describes the equilibrium chem- terms of activities. An equilibrium constant greater
ical mass activities of a reversible reaction. The rate of than unity suggests that equilibrium lies to the right-
reaction is proportional to the effective concentration hand side of the equation describing the chemical
of the reacting substances. For the reaction: reaction and that the forward reaction is favoured.